推薦產品
等級
pharmaceutical primary standard
API 家族
aminoglutethimide
製造商/商標名
EDQM
mp
152-154 °C (lit.)
應用
pharmaceutical (small molecule)
格式
neat
儲存溫度
2-8°C
SMILES 字串
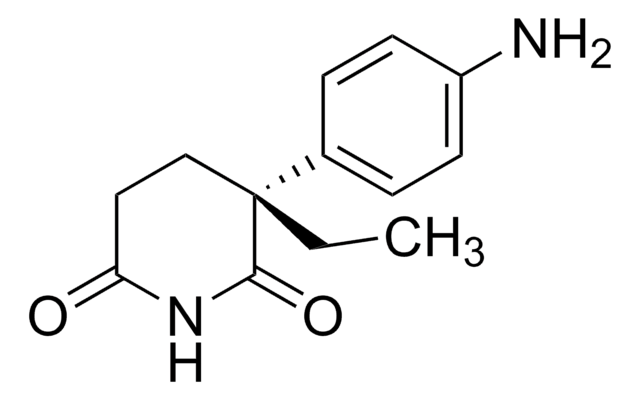
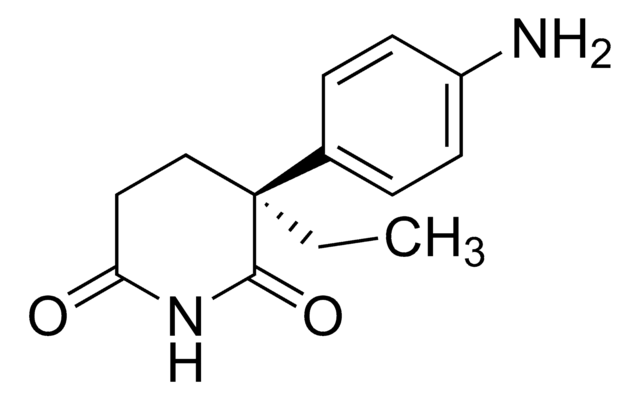
CCC1(CCC(=O)NC1=O)c2ccc(N)cc2
InChI
1S/C13H16N2O2/c1-2-13(8-7-11(16)15-12(13)17)9-3-5-10(14)6-4-9/h3-6H,2,7-8,14H2,1H3,(H,15,16,17)
InChI 密鑰
ROBVIMPUHSLWNV-UHFFFAOYSA-N
基因資訊
human ... CYP11A1(1583) , CYP19A1(1588)
尋找類似的產品? 前往 產品比較指南
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Aminoglutethimide EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
生化/生理作用
DL-Aminoglutethimide is a derivative of the sedative glutethimide. Originally introduced as an anticonvulsant, it was found to cause adrenal insufficiency. Blocks adrenal steroidogenesis by inhibiting the enzymatic conversion of cholesterol to pregnenolone. It also blocks the peripheral conversion (aromatization) of androgenic precursors to estrogens. The D-isomer is 30 times more potent at inhibiting aromatase activity, whereas the L-isomer is more potent at inhibiting cholesterol side-chain cleavage (steroidogenesis).
包裝
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
其他說明
Sales restrictions may apply.
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
P E Lønning et al.
Drugs, 35(6), 685-710 (1988-06-01)
During the last decade aminoglutethimide has been recognised as a valuable alternative in endocrine therapy for advanced breast cancer. Although some side effects do occur, most often these are initial effects which subside within a few weeks, and cessation of
R J Santen
Breast cancer research and treatment, 1(3), 183-202 (1981-01-01)
Fifty to sixty percent of postmenopausal women with estrogen receptor positive metastatic breast cancer respond objectively to surgical ablation of the pituitary or adrenal glands. Several investigators have recently developed medical alternatives to surgical ablative therapy for these patients. This
Aminoglutethimide: a "side-effect" turned to therapeutic advantage.
S W Hughss et al.
Postgraduate medical journal, 46(537), 409-416 (1970-07-01)
Aminoglutethimide in the treatment of advanced breast cancer.
R C Stuart-Harris et al.
Cancer treatment reviews, 11(3), 189-204 (1984-09-01)
A M Brodie et al.
Critical reviews in oncology/hematology, 5(4), 361-396 (1986-01-01)
Approximately one third of human breast carcinomas are hormone dependent and regress upon reduction of circulating estrogen levels. Traditional treatment strategies utilized surgical ablative methods to lower estrogen concentrations as treatment of breast cancer. Currently, investigative emphasis is focused upon
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務