推薦產品
化驗
≥98%
形狀
powder
反應適用性
reaction type: solution phase peptide synthesis
存貨情形
available only in USA
儲存溫度
2-8°C
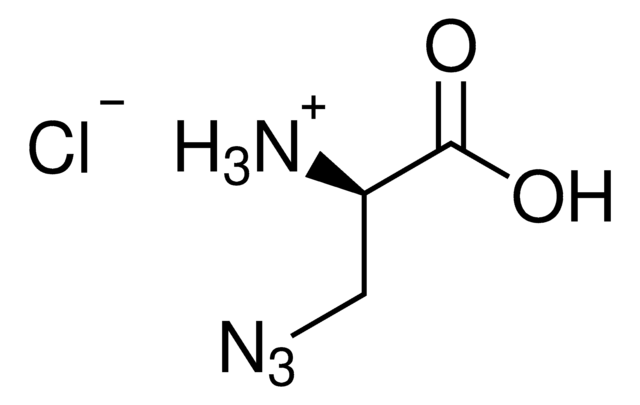
InChI
1S/C4H8N4O2.ClH/c5-3(4(9)10)1-2-7-8-6;/h3H,1-2,5H2,(H,9,10);1H/t3-;/m0./s1
InChI 密鑰
MHHYJRIDKLZZEO-DFWYDOINSA-N
應用
The in vivo incorporation of clickable unnatural amino acids such as 4-Azido-L-homoalanine with unique reactivity at a defined postition is used for functionalization of proteins. The azide functionalities in the protein can then be modified with almost any alkyne bearing molecule by Cu(I)-catalyzed azide-alkyne cycloaddition (CuAAC). Copper-free alternatives with strained internal alkynes are also available (SPAAC). Some examples enabled with this technique are protein PEGylation, masking with sugars, and the attachment to antibodies.
訊號詞
Danger
危險聲明
危險分類
Self-react. C
儲存類別代碼
5.2 - Organic peroxides and self-reacting hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Jigang Wang et al.
Nature protocols, 12(2), 279-288 (2017-01-13)
At present, several assays that use radioisotope labeling to quantify the degradation of long-lived proteins have been developed to measure autophagic flux. Here, we describe a nonradioactive pulse-chase protocol using L-azidohomoalanine (AHA) labeling to quantify long-lived protein degradation during autophagy.
Milena Ullrich et al.
Nature protocols, 9(9), 2237-2255 (2014-08-29)
In this protocol we describe the incorporation of bio-orthogonal amino acids as a versatile method for visualizing and identifying de novo-synthesized proteins in the roundworm Caenorhabditis elegans. This protocol contains directions on implementing three complementary types of analysis: 'click chemistry'
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務






![[3-(2-carboxyethyl)phenyl]boronic acid AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/265/067/68263dcf-5afc-49a6-982b-0394e48bf9c2/640/68263dcf-5afc-49a6-982b-0394e48bf9c2.png)
