推薦產品
等級
analytical standard
儲存期限
limited shelf life, expiry date on the label
技術
HPLC: suitable
gas chromatography (GC): suitable
折射率
n20/D 1.582 (lit.)
bp
89 °C/16 mmHg (lit.)
mp
−8 °C (lit.)
密度
0.997 g/mL at 25 °C (lit.)
應用
environmental
格式
neat
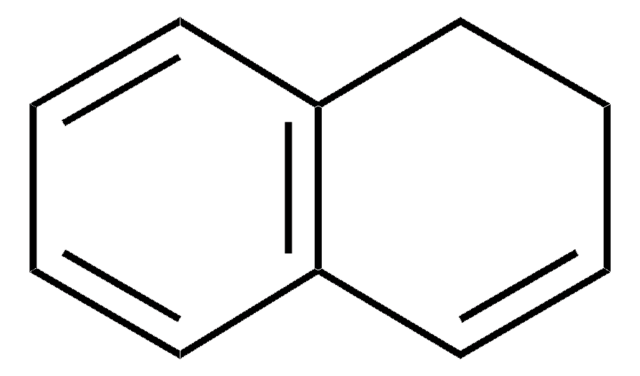
SMILES 字串
C1Cc2ccccc2C=C1
InChI
1S/C10H10/c1-2-6-10-8-4-3-7-9(10)5-1/h1-3,5-7H,4,8H2
InChI 密鑰
KEIFWROAQVVDBN-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
1,2-Dihydronaphthalene is a bicyclic hydrocarbon, which resembles naphthalene but shows partial unsaturation in one of its rings. Its derivatives find wide applications in natural compounds of therapeutic interest.
應用
1,2-Dihydronaphthalene may be used as an analytical standard for the determination of the analyte in polycyclic aromatic hydrocarbon (PAH) mixtures by gas chromatography technique.
Refer to the product′s Certificate of Analysis for more information on a suitable instrument technique. Contact Technical Service for further support.
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
152.6 °F - closed cup
閃點(°C)
67 °C - closed cup
個人防護裝備
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Prediction of gas chromatographic retention indexes of polycyclic aromation compounds and nitrated polycyclic aromatic compounds
Rohrbaugh RH and Jurs PC
Analytical Chemistry, 58(6), 1210-1212 (1986)
The synthesis of novel dihydronaphthalenes and benzofluorenes
Novel Selenium-Mediated Rearrangements and Cyclisations, 77(6), 1210-1212 (2012)
Retention indices for programmed-temperature capillary-column gas chromatography of polycyclic aromatic hydrocarbons
Lee ML, et al.
Analytical Chemistry, 51(6), 768-773 (1979)
D S Torok et al.
Journal of bacteriology, 177(20), 5799-5805 (1995-10-01)
Bacterial strains expressing toluene and naphthalene dioxygenase were used to examine the sequence of reactions involved in the oxidation of 1,2-dihydronaphthalene. Toluene dioxygenase of Pseudomonas putida F39/D oxidizes 1,2-dihydronaphthalene to (+)-cis-(1S,2R)-dihydroxy-1,2,3,4-tetrahydronaphthalene, (+)-(1R)-hydroxy-1,2-dihydronaphthalene, and (+)-cis-(1R,2S)-dihydroxy-1,2-dihydronaphthalene. In contrast, naphthalene dioxygenase of Pseudomonas
S L Eaton et al.
Applied and environmental microbiology, 62(12), 4388-4394 (1996-12-01)
The substrate oxidation profiles of Sphingomonas yanoikuyae B1 biphenyl-2,3-dioxygenase and cis-biphenyl dihydrodiol dehydrogenase activities were examined with 1,2-dihydronaphthalene and various cis-diols as substrates. m-Xylene-induced cells of strain B1 oxidized 1,2-dihydronaphthalene to (-)-(1R,2S)-cis-1,2-dihydroxy-1,2-3,4-tetrahydronaphthalene as the major product (73% relative yield). Small
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務