推薦產品
化驗
≥99% (HPLC)
品質等級
形狀
powder
效力
70 nM Ki
製造商/商標名
Calbiochem®
儲存條件
OK to freeze
protect from light
顏色
off-white
溶解度
DMSO: 100 mg/mL
儲存溫度
2-8°C
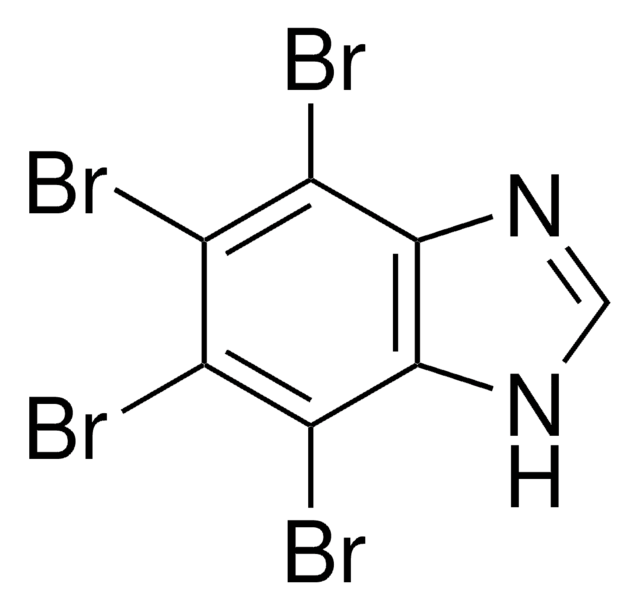
InChI
1S/C7H2Br4N2/c8-2-3(9)5(11)7-6(4(2)10)12-1-13-7/h1H,(H,12,13)
InChI 密鑰
LOEIRDBRYBHAJB-UHFFFAOYSA-N
一般說明
A cell-permeable halogenated benzimidazole compound that acts as a selective and ATP competitive inhibitor of casein kinase II (CK2, Ki = 70 nM for the active hetero-tetrameric form). However, its effect on free CK2a and CK2a′ subunits is significantly reduced (Ki = 510 and 400 nM, respectively). Reported to reduce viability and proliferation in HeLa, Jurkat, and HL-60 cells (at ˜25 µM). Also shown to disrupt the promoter activity of CYP24A1 and down-regulates its endogenous and 1, 25-Vitamin D3 (1,25-D3)-induced expression in PC3 cells. Synergistically enhances the anti-tumor effect of 1, 25-D3 in a PC3 xenograft murine model.
生化/生理作用
Primary Target
CK2
CK2
Reversible: yes
包裝
Packaged under inert gas
警告
Toxicity: Standard Handling (A)
重構
Following reconstitution, aliquot and freeze (-20°C). Stock solutions are stable for up to 3 months at -20°C.
其他說明
Szyszka, R. et al. 1995. Biochem. Biophys. Res. Comm.208, 418.
Zien, P. et al. 2003. Biochem. Biophys. Res. Comm.306, 129.
Zien, P. et al. 2003. Biochem. Biophys. Res. Comm.312, 623.
Zien, P. et al. 2005. Biochim. Biophy. Acta1754, 271.
Luo, W. et al. 2013. Cancer Res.73, 2289.
Zien, P. et al. 2003. Biochem. Biophys. Res. Comm.306, 129.
Zien, P. et al. 2003. Biochem. Biophys. Res. Comm.312, 623.
Zien, P. et al. 2005. Biochim. Biophy. Acta1754, 271.
Luo, W. et al. 2013. Cancer Res.73, 2289.
法律資訊
CALBIOCHEM is a registered trademark of Merck KGaA, Darmstadt, Germany
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
分析證明 (COA)
輸入產品批次/批號來搜索 分析證明 (COA)。在產品’s標籤上找到批次和批號,寫有 ‘Lot’或‘Batch’.。
Piotr Zień et al.
Biochemical and biophysical research communications, 312(3), 623-628 (2003-12-19)
Two ATP-competitive inhibitors-4,5,6,7-tetrabromo-benzotriazole (TBBt) and 4,5,6,7-tetrabromo-benzimidazole (TBBz) have been shown to decrease activity of CK2 holoenzyme. Surprisingly it occurs that TBBz contrary to TBBt does not inhibit free catalytic subunit CK2 [Formula: see text]. Both inhibitors are virtually inactive against
Piotr Zien et al.
Biochimica et biophysica acta, 1754(1-2), 271-280 (2005-10-06)
The development of selective cell-permeable inhibitors of protein kinase CK2 has represented an important advance in the field. However, it is important to not overlook the existence of discrete molecular forms of CK2 that arise from the presence of distinct
Wei Luo et al.
Cancer research, 73(7), 2289-2297 (2013-01-30)
Vitamin D has broad range of physiological functions and antitumor effects. 24-Hydroxylase, encoded by the CYP24A1 gene, is the key enzyme for degrading many forms of vitamin D including the most active form, 1,25D(3). Inhibition of CYP24A1 enhances 1,25D(3) antitumor
Piotr Zień et al.
Biochemical and biophysical research communications, 306(1), 129-133 (2003-06-06)
Like the previously reported 4,5,6,7-tetrabromobenzotriazole (TBBt), the structurally related 4,5,6,7-tetrabromobenzimidazole (TBBz) is a selective ATP-competitive inhibitor of protein kinase CK2 from such divergent sources as yeast, rat liver, Neurospora crassa and Candida tropicalis, with K(i) values in the range 0.5-1
R Szyszka et al.
Biochemical and biophysical research communications, 208(1), 418-424 (1995-03-08)
Several halogeno benzimidazole riboside inhibitors of animal and plant protein kinases CK I and CK II (also known as casein kinases I and II), were found to be effective inhibitors of Saccharomyces cerevisiae CK II, but not of the 27-kDa
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務