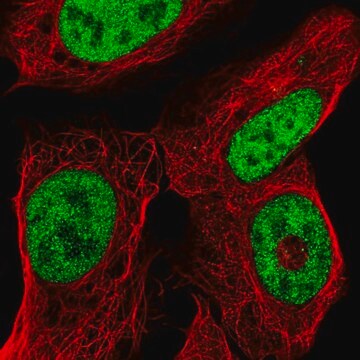
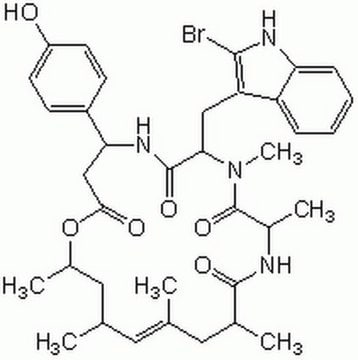
A cell-permeable I-BET (Cat. No.
401010) class of thienodiazepine derivative whose (
S)-(+), but not (
R)-(-), enantiomer is shown to target both bromodomains (BD1 & BD2) of BET (bromodomain and extra terminal) family members BRD2 (K
D = 128.4 nM for human BD1; Δ
Tmobs = 6.47 and 7.97 °C, respectively, for human BD1 and BD2), BRD3 (K
D = 59.5 and 82.0 nM, respectively, for human BD1 and BD2), BRD4 (K
D = 49.0 and 90.1 nM, respectively, for human BD1 and BD2), and BRD6/BRDT (K
D = 190.1, 44.1, 77.5, and 59.2 nM, respectively, for human BD1, human BD2, murine BD1, and murine BD2) in a Kac- (ε-N-acetylated lysine) competitive manner, exhibiting little affinity toward 23 other BD-containing proteins, including the single BD-containing BRD1 and BRD9, and little or no activity against a panel of more than 50 receptors, ion channels, and transporters. Effectively inhibits the oncogenic BRD4-NUT (Nuclear protein in testis) fusion protein-dependent NMC (NUT midline carcinoma) and c-Myc oncoprotein-dependent MM (multiple myeloma) proliferation both in cultures (IC
50<1 M)
in vitro and in mice (50 mg/kg/day i.p.)
in vivo. Also reported to cross the blood-testis boundary in male mice and effectively reduce testis mass, sperm count and motility (50 mg/kg; b.i.d. i.p.) in a reversible manner by targeting BRDT-mediated spermatogenesis without interfering hormone levels.
A cell-permeable I-BET (Cat. No.
401010) class of thienodiazepine derivative whose (
S)-(+), but not (
R)-(-), enantiomer is shown to target both bromodomains (BD1 & BD2) of BET family members BRD2, BRD3, BRD4, and BRD6/BRDT in a Kac- (ε-N-acetylated lysine) competitive manner, exhibiting little affinity toward 23 other BD-containing proteins, BRD1 and BRD9, and little or no activity against a panel of more than 50 receptors, ion channels, and transporters. Effectively inhibits the oncogenic BRD4-NUT fusion-dependent NUT midline carcinoma and c-Myc oncoprotein-dependent multiple myeloma proliferation both in cultures (IC
50<1 M)
in vitro and in mice (50 mg/kg/day i.p.)
in vivo. Also reported to cross the blood-testis boundary in male mice and effectively block BRDT-mediated spermatogenesis without affecting hormone levels. The set contains 5 mg of the active (
S)-(+)-JQ1 (540696) and 5 mg of the inactive (
R)-(-) enantiomer (500585).