推薦產品
形狀
methylene chloride solution
包裝
pkg of 1 × 1 mL (870818M-10mg)
製造商/商標名
Avanti Research™ - A Croda Brand 870818M
脂質類型
neutral glycerides
neutral lipids
運輸包裝
dry ice
儲存溫度
−20°C
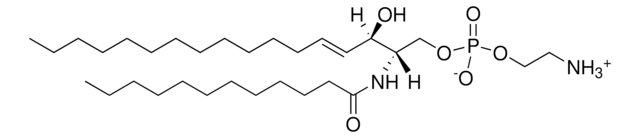
SMILES 字串
O=C(CCC/C=C\C/C=C\C/C=C\C/C=C\CCCCC)NCC(O)=O
InChI
1S/C22H35NO3/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-21(24)23-20-22(25)26/h6-7,9-10,12-13,15-16H,2-5,8,11,14,17-20H2,1H3,(H,23,24)(H,25,26)/b7-6-,10-9-,13-12-,16-15-
InChI 密鑰
YLEARPUNMCCKMP-DOFZRALJSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
N-arachidonoylglycine (NAgly) is an endogenous ligand and a signaling lipid. It belongs to the eicosanoid family. NAgly is a natural ligand to activate G-protein coupled receptor, namely GPR18. It is found in rat brain and spinal cord.
生化/生理作用
N-arachidonoylglycine (NAgly) exhibits analgesic effects.
包裝
2 mL Amber Glass Sealed Ampule (870818M-10mg)
法律資訊
Avanti Research is a trademark of Avanti Polar Lipids, LLC
訊號詞
Warning
危險分類
Carc. 2 - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Central nervous system
水污染物質分類(WGK)
WGK 2
分析證明 (COA)
輸入產品批次/批號來搜索 分析證明 (COA)。在產品’s標籤上找到批次和批號,寫有 ‘Lot’或‘Batch’.。
S M Huang et al.
The Journal of biological chemistry, 276(46), 42639-42644 (2001-08-24)
In mammals, specific lipids and amino acids serve as crucial signaling molecules. In bacteria, conjugates of lipids and amino acids (referred to as lipoamino acids) have been identified and found to possess biological activity. Here, we report that mammals also
Resolution of inflammation by N-arachidonoylglycine
Burstein S H, et al.
Journal of Cellular Biochemistry, 112(11), 3227-3233 (2011)
S H Burstein et al.
Prostaglandins & other lipid mediators, 61(1-2), 29-41 (2000-04-29)
In addition to the well studied hydrolytic metabolism of anandamide, a number of oxidative processes are also possible. Several routes somewhat analogous to the metabolism of free arachidonic acid have been reported. These involve mediation by various lipoxygenases and COX-2
T Sheskin et al.
Journal of medicinal chemistry, 40(5), 659-667 (1997-02-28)
In order to establish the structural requirements for binding to the brain cannabinoid receptor (CB1), we have synthesized numerous fatty acid amides, ethanolamides, and some related simple derivatives and have determined their Ki values. A few alpha-methyl- or alpha, alpha-dimethylarachidonoylalkylamides
Identification of N-arachidonylglycine as the endogenous ligand for orphan G-protein-coupled receptor GPR18
Kohno M, et al.
Biochemical and Biophysical Research Communications, 347(3), 827-832 (2006)
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務