933678
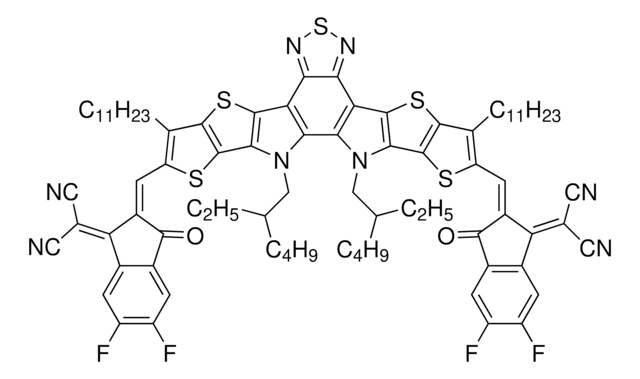
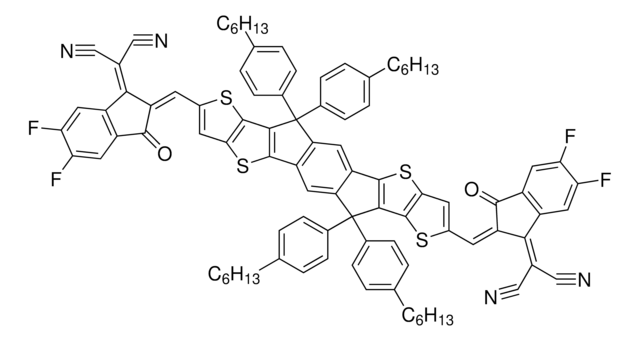
Y6(BTPTT-4F)
同義詞:
Y6, 2,2′-((2Z,2′Z)-((12,13-Bis(2-ethylhexyl)-3,9-diundecyl-12,13-dihydro-[1,2,5]thiadiazolo[3,4-e]thieno-[2",3":4′,5′]thieno[2′,3′:4,5]pyrrolo[3,2-g]thieno-[2′,3′:4,5]thieno[3,2-b]indole-2,10-diyl)bis(methanylylidene))-bis(5,6-difluoro-3-oxo-2,3-dihydro-1H-indene-2,1-diylidene))dimalononitrile
About This Item
推薦產品
化驗
≥99% (H-NMR)
品質等級
顏色
black
溶解度
chloroform: soluble
λmax
731 nm in chloroform (UV)
軌道能量
HOMO - 5.65 eV
LUMO - 4.1 eV
SMILES 字串
Fc1cc2c(cc1F)C(=C(C#N)C#N)\C(=C\c3[s]c4c([s]c5c4[n](c6c7[n](c8c([s]c%10c8[s]c(c%10CCCCCCCCCCC)\C=C%11/C(=O)c%12c(cc(c(c%12)F)F)C/%11=C(C#N)C#N)c7c9n[s]nc9c65)CC(CCCC)CC)CC(CCCC)CC)c3CCCCCCCCCCC)\C2=O
InChI
1S/C82H86F4N8O2S5/c1-7-13-17-19-21-23-25-27-29-33-51-63(39-57-65(49(41-87)42-88)53-35-59(83)61(85)37-55(53)75(57)95)97-81-73-79(99-77(51)81)67-69-70(92-101-91-69)68-72(71(67)93(73)45-47(11-5)31-15-9-3)94(46-48(12-6)32-16-10-4)74-80(68)100-78-52(34-30-28-2
InChI 密鑰
XJRVXAOYOOOQLU-LAGONYLDSA-N
尋找類似的產品? 前往 產品比較指南
應用
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務