推薦產品
等級
sublimed grade
品質等級
描述
μe ≈ 1.0 x 10-3 cm2 V−1 s−1
化驗
≥99% (HPLC)
損耗
0.5% TGA, > 310 °C (weight loss)
mp
195-200 °C
轉變溫度
Tg >310 °C ((0.5% weight loss))
溶解度
chloroform: soluble
dichloromethane: soluble
螢光
λem 353 nm in dichloromethane (PL)
軌道能量
HOMO 6.75 eV
LUMO 2.75 eV
&lambda ;
in dichloromethane
紫外吸收
λ: 254 nm Amax
SMILES 字串
C1(C2=CC=CC(C3=CN=CC=C3)=C2)=CC(C4=CC=CC(C5=CN=CC=C5)=C4)=CC(C6=CC=CC(C7=CN=CC=C7)=C6)=C1
InChI
1S/C39H27N3/c1-7-28(34-13-4-16-40-25-34)19-31(10-1)37-22-38(32-11-2-8-29(20-32)35-14-5-17-41-26-35)24-39(23-37)33-12-3-9-30(21-33)36-15-6-18-42-27-36/h1-27H
InChI 密鑰
CINYXYWQPZSTOT-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
應用
1,3,5-Tri[(3-pyridyl)-phen-3-yl]benzene, also known as TmPyPB, is a solution-processable electron transport / hole blocking layer (ETL / HBL) material used in organic electronics. It has a μe around 1.0 x 10-3 cm2 V−1 s−1.
1,3,5-Tri[(3-pyridyl)phen-3-yl]benzene can be employed as a component in the synthesis of luminescent materials, including organic light-emitting diodes (OLEDs) or fluorescent dyes for sensing and imaging applications. It an be used as a building block or donor material in the active layer of Organic photovoltaics (OPV) devices.
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
分析證明 (COA)
輸入產品批次/批號來搜索 分析證明 (COA)。在產品’s標籤上找到批次和批號,寫有 ‘Lot’或‘Batch’.。
Huijun Liu et al.
Angewandte Chemie (International ed. in English), 57(30), 9290-9294 (2018-06-02)
Non-doped organic light-emitting diodes (OLEDs) possess merits of higher stability and easier fabrication than doped devices. However, luminescent materials with high exciton use are generally unsuitable for non-doped OLEDs because of severe emission quenching and exciton annihilation in neat films.
Meng Li et al.
Angewandte Chemie (International ed. in English), 57(11), 2889-2893 (2018-01-23)
Aromatic-imide-based thermally activated delayed fluorescent (TADF) enantiomers, (+)-(S,S)-CAI-Cz and (-)-(R,R)-CAI-Cz, were efficiently synthesized by introducing a chiral 1,2-diaminocyclohexane to the achiral TADF unit. The TADF enantiomers exhibited high PLQYs of up to 98 %, small ΔEST values of 0.06 eV, as well
Wei Li et al.
Angewandte Chemie (International ed. in English), 58(2), 582-586 (2018-11-21)
To date, blue dual fluorescence emission (DFE) has not been realized because of the limited choice of chemical moieties and severe geometric deformation of the DFE emitters leading to strong intramolecular charge transfer (ICT) with a large Stokes shift in
Hui Wang et al.
Advanced materials (Deerfield Beach, Fla.), 26(30), 5198-5204 (2014-06-07)
Thermally activated delayed fluorescence emitters with small energy gap between the triplet and singlet (ΔEST ), TXO-PhCz and TXO-TPA, have been successfully synthesized by combining a hole-transporting TPA/PhCz moiety and an electron-transporting TXO moiety. Both compounds display efficient solid-state luminescence
Wei Li et al.
Angewandte Chemie (International ed. in English), 58(33), 11301-11305 (2019-06-14)
Blue thermally activated delayed fluorescence (TADF) emitters that can simultaneously achieve high efficiency in doped and nondoped organic light-emitting diodes (OLEDs) are rarely reported. Reported here is a strategy using a tri-spiral donor for such versatile blue TADF emitters. Impressively
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務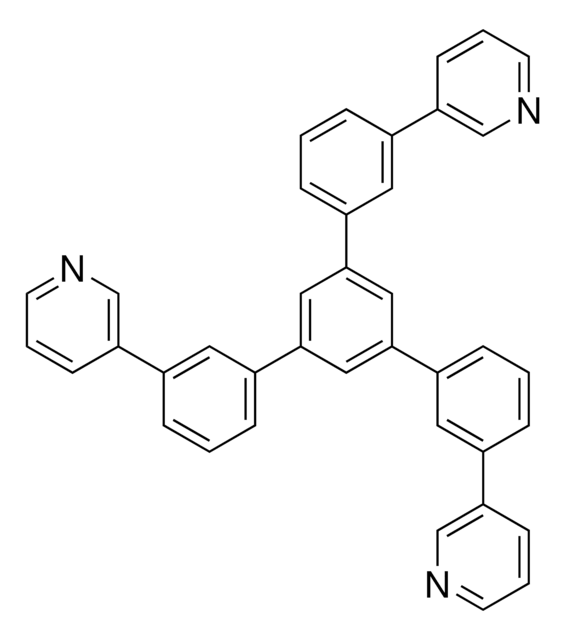





![4,4′-环己烯[N,N-双(4-甲基苯基)苯胺] ≥97% (HPLC)](/deepweb/assets/sigmaaldrich/product/structures/111/787/16bde1ce-c76d-46d6-9e1f-9ce09f82d038/640/16bde1ce-c76d-46d6-9e1f-9ce09f82d038.png)