推薦產品
化驗
≥95%
形狀
powder
儲存溫度
−20°C
InChI
1S/C19H29NO4/c1-5-23-18(21)15-12(3)20-13(4)16(19(22)24-6-2)17(15)14-10-8-7-9-11-14/h14,17,20H,5-11H2,1-4H3
InChI 密鑰
GERWBKSVDHUVIT-UHFFFAOYSA-N
應用
Diethyl-4-cyclohexyl-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate was reported to chemoselectively modify histidine under visible light where the unsubstituted nitrogen groups on the modified His imidazole are conserved. Diethyl-4-cyclohexyl-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate is also a versitile reagent for photoredox chemistry.
Product can be used with our line of photoreactors: Including Penn PhD (Z744035) & SynLED 2.0 (Z744080)
Product can be used with our line of photoreactors: Including Penn PhD (Z744035) & SynLED 2.0 (Z744080)
其他說明
Histidine-specific peptide modification via visible-light-promoted C-H alkylation
Open-Air Alkylation Reactions in Photoredox-Catalyzed DNA-Encoded Library Synthesis
A photocatalyst-free photo-induced denitroalkylation of ß-nitrostyrenes with 4-alkyl substituted Hantzsch esters at room temperature
Intermolecular Radical Addition to Ketoacids Enabled by Boron Activation
Oxa- and Azabenzonorbornadienes as Electrophilic Partners under Photoredox/Nickel Dual Catalysis
Exploration of a chiral cobalt catalyst for visible-light-induced enantioselective radical conjugate addition
Open-Air Alkylation Reactions in Photoredox-Catalyzed DNA-Encoded Library Synthesis
A photocatalyst-free photo-induced denitroalkylation of ß-nitrostyrenes with 4-alkyl substituted Hantzsch esters at room temperature
Intermolecular Radical Addition to Ketoacids Enabled by Boron Activation
Oxa- and Azabenzonorbornadienes as Electrophilic Partners under Photoredox/Nickel Dual Catalysis
Exploration of a chiral cobalt catalyst for visible-light-induced enantioselective radical conjugate addition
訊號詞
Warning
危險分類
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
分析證明 (COA)
輸入產品批次/批號來搜索 分析證明 (COA)。在產品’s標籤上找到批次和批號,寫有 ‘Lot’或‘Batch’.。
Xuefeng Wang et al.
Chemical communications (Cambridge, England), 55(43), 6010-6013 (2019-05-08)
A sulfonylation reaction of 4-substituted Hantzsch esters, DABCO·(SO2)2, and electron-deficient alkenes at room temperature in the presence of photoredox catalysis under visible light irradiation is described. Not only (E)-chalcones but also (vinylsulfonyl)benzene and 2-vinylpyridine are all suitable substrates in the
Kaiqian Wang et al.
Organic & biomolecular chemistry, 17(15), 3845-3852 (2019-04-03)
Herein, we report a simple, economical, and effective acid-mediated method for the in situ deuteration of Hantzsch esters and their 4-substituted derivatives, including some drugs that constitute important calcium channel blockers which are effective for hypertension treatment. Hydrogen isotope exchange
Jennifer K Matsui et al.
Angewandte Chemie (International ed. in English), 57(48), 15847-15851 (2018-10-12)
A regioselective, nickel-catalyzed photoredox allylation of secondary, benzyl, and α-alkoxy radical precursors is disclosed. Through this manifold, a variety of linear allylic alcohols and allylated monosaccharides are accessible in high yields under mild reaction conditions. Quantum mechanical calculations [DFT and
Hai-Wu Du et al.
Organic letters, 22(4), 1542-1546 (2020-01-29)
In this study, a facile and efficient method to synthesize monofluoroalkenes by photoredox catalytic defluorinative alkylation of gem-difluoroalkenes with 4-alkyl-1,4-dihydropyridines under mild conditions (room temperature) is described. This novel strategy is applicable for a broad range of gem-difluoroalkene substrates with
Kai Zhang et al.
Angewandte Chemie (International ed. in English), 58(38), 13375-13379 (2019-07-12)
Chiral catalysts tolerating photochemical reactions are in great demand for the vast development of visible-light-induced asymmetric synthesis. Now, chiral octahedral complexes based on earth-abundant metal and chiral N4 ligands are reported. One well-defined chiral CoII -complex is shown to be
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務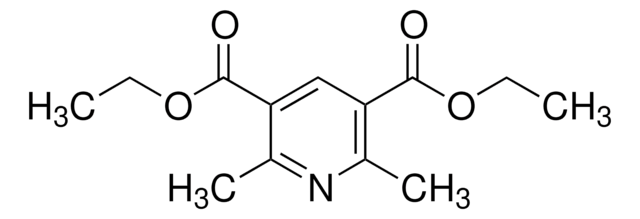






![[Ir(dtbbpy)(ppy)2]PF6](/deepweb/assets/sigmaaldrich/product/structures/158/329/2544d673-d267-4aa1-8f46-2652aad4bfa0/640/2544d673-d267-4aa1-8f46-2652aad4bfa0.png)

