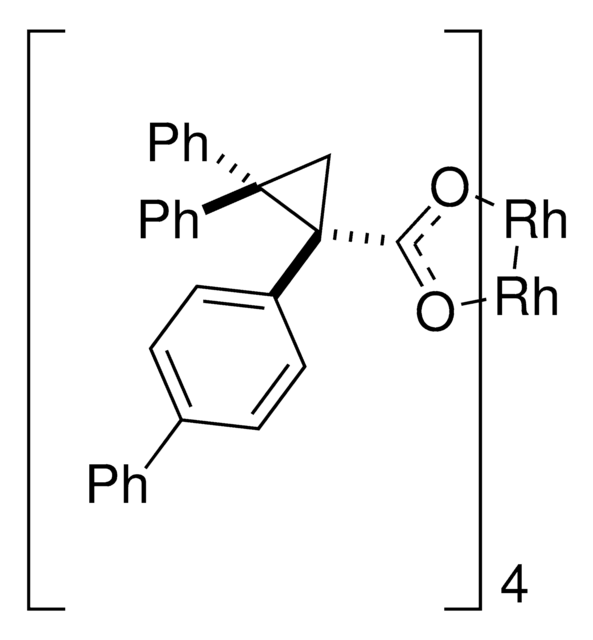
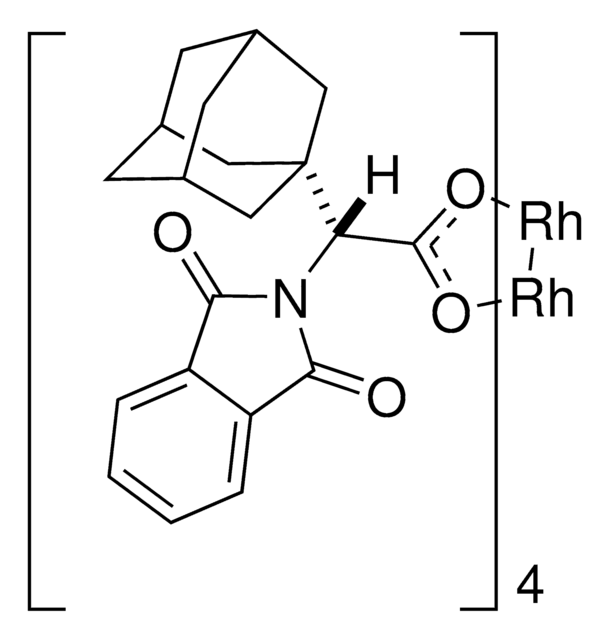
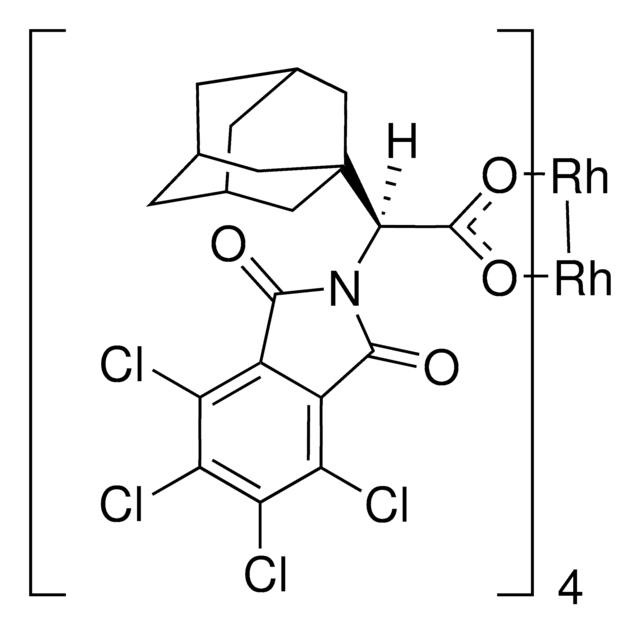
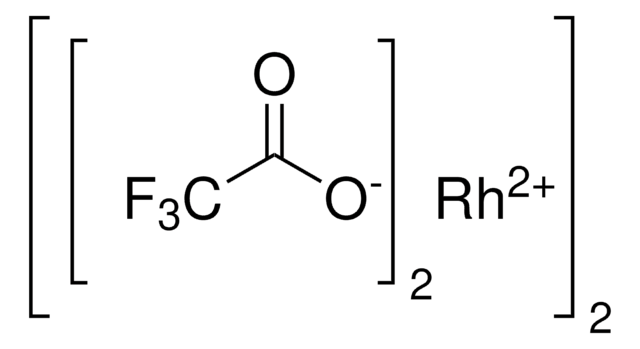
推薦產品
形狀
powder or crystals
mp
>300 °C
InChI
1S/4C20H21NO4.2Rh/c4*22-17-14-3-1-2-4-15(14)18(23)21(17)16(19(24)25)20-8-11-5-12(9-20)7-13(6-11)10-20;;/h4*1-4,11-13,16H,5-10H2,(H,24,25);;/q;;;;2*+2/p-4/t4*11-,12+,13-,16-,20?;;/m1111../s1
InChI 密鑰
SGEDWOHAUXKUGM-PAPHCAFZSA-J
相關類別
應用
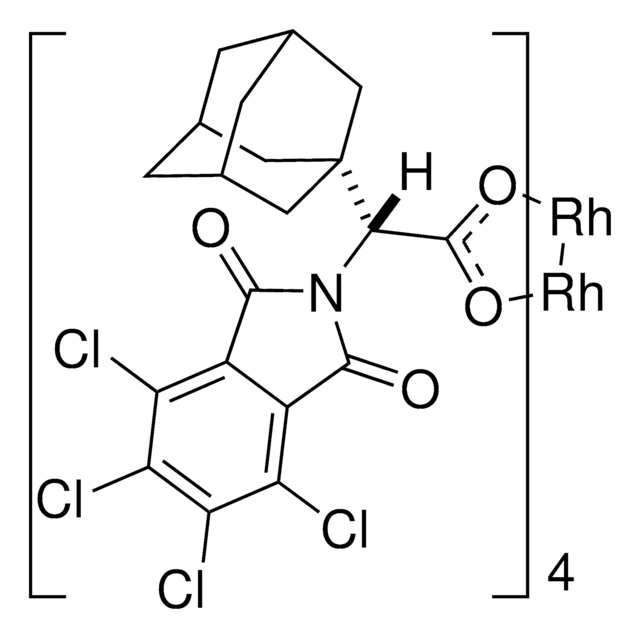
这种手性Rh(II)二聚体是在Davies实验室开发的,可通过高区域和立体控制进行不对称的卡宾和氮烯反应(C-H插入,环丙烷化,氮杂环丙烷化,C-H胺化)。
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
分析證明 (COA)
輸入產品批次/批號來搜索 分析證明 (COA)。在產品’s標籤上找到批次和批號,寫有 ‘Lot’或‘Batch’.。
Justin R Denton et al.
Organic letters, 11(4), 787-790 (2009-01-17)
The reaction of a variety of alpha-aryl-alpha-diazo ketones with activated olefins, catalyzed by the adamantyl glycine-derived dirhodium complex Rh(2)(S-PTAD)(4), generates cyclopropyl ketones with high diastereoselectivity (up to >95:5 dr) and enantioselectivity (up to 98% ee). Intermolecular C-H functionalization of 1,4-cyclohexadiene
Zhanjie Li et al.
Journal of the American Chemical Society, 132(1), 396-401 (2009-12-10)
The rhodium-catalyzed reaction of racemic allyl alcohols with methyl phenyldiazoacetate or methyl styryldiazoacetate results in a two-step process, an initial oxonium ylide formation followed by a [2,3]-sigmatropic rearrangement. This process competes favorably with the more conventional O-H insertion chemistry as
Kuangbiao Liao et al.
Nature, 533(7602), 230-234 (2016-05-14)
The laboratory synthesis of complex organic molecules relies heavily on the introduction and manipulation of functional groups, such as carbon-oxygen or carbon-halogen bonds; carbon-hydrogen bonds are far less reactive and harder to functionalize selectively. The idea of C-H functionalization, in
Yan Su et al.
Organic letters, 18(17), 4356-4359 (2016-08-17)
Optically active cis-cyclopropane carboxylates are prepared via the Rh2(S-PTAD)4-catalyzed cyclopropanation of α-silyl styrenes with aryl diazoacetates followed by desilylation of the resulting silyl cyclopropane carboxylates. The conjugation of the aryl ring with C═C bond and π stacking are proposed for
Cristian Soldi et al.
Journal of the American Chemical Society, 136(43), 15142-15145 (2014-10-14)
The first asymmetric insertion reactions of donor-donor carbenoids, i.e., those with no pendant electron-withdrawing groups, are reported. This process enables the synthesis of densely substituted benzodihydrofurans with high levels of enantio- and diastereoselectivity. Preliminary results show similar efficiency in the
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務
![双[(α,α,α′,α′-四甲基-1,3-苯二丙酸)铑] 95%](/deepweb/assets/sigmaaldrich/product/structures/102/178/d1171a49-0358-406b-8b32-04324dbf9c02/640/d1171a49-0358-406b-8b32-04324dbf9c02.png)