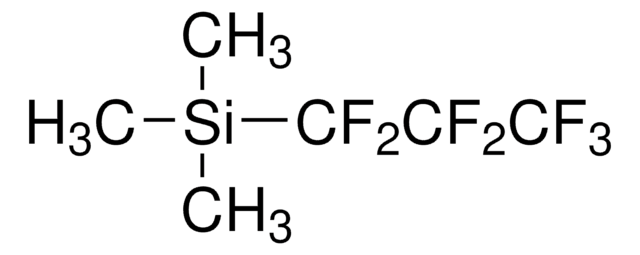全部照片(1)
About This Item
經驗公式(希爾表示法):
C5H9F5Si
CAS號碼:
分子量::
192.20
MDL號碼:
分類程式碼代碼:
12352101
PubChem物質ID:
NACRES:
NA.22
推薦產品
化驗
97%
形狀
liquid
折射率
n/D 1.325
密度
1.095 g/mL
SMILES 字串
C[Si](C)(C)C(F)(F)C(F)(F)F
InChI
1S/C5H9F5Si/c1-11(2,3)5(9,10)4(6,7)8/h1-3H3
InChI 密鑰
MTPVUVINMAGMJL-UHFFFAOYSA-N
一般說明
Trimethylpentafluoroethylsilane ((Pentafluoroethyl)trimethylsilane) is a perfluoroalkylsilane. Alkyl triflates undergo nucleophilic pentafluoroethylation with trimethylpentafluoroethylsilane to form the corresponding pentafluoroethylated alkanes.
應用
Trimethylpentafluoroethylsilane ((Pentafluoroethyl)trimethylsilane) may be used as a perfluoroalkylating reagent for the synthesis of the following quinoline derivatives:
- 5-bromo-2-(perfluoroethyl)quinoline
- 8-methoxy-2-(perfluoroethyl)quinoline
- 1-(perfluoroethyl)isoquinoline
- 8-(tert-butoxy)-5,7-dichloro-2-(perfluoroethyl)quinolone
Trimethylpentafluoroethylsilane has been reported as a Ruppert-Prakash type reagent for the addition of pentafluoroethane. Recent report by Larionov and coworkers displayed the feasibility of adding pentrafluoroethane to N-heterocycles under basic conditions.
訊號詞
Danger
危險分類
Eye Irrit. 2 - Flam. Liq. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
3 - Flammable liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
1.4 °F - closed cup - (calculated)
閃點(°C)
-17 °C - closed cup - (calculated)
分析證明 (COA)
輸入產品批次/批號來搜索 分析證明 (COA)。在產品’s標籤上找到批次和批號,寫有 ‘Lot’或‘Batch’.。
A Facile New Method for the Two-step Substitution of Hydroxy Groups in Primary Alcohols for Trifluoromethyl and Pentafluoroethyl Moieties.
Sevenard DV, et al.
Synlett, 2001(03), 0379-0381 (2001)
Experimental determination of the conformational free energies (A values) of fluorinated substituents in cyclohexane by dynamic 19 F NMR spectroscopy. Part 2. Extension to fluoromethyl, difluoromethyl, pentafluoroethyl, trifluoromethylthio and trifluoromethoxy groups.
Carcenac Y, et al.
New. J. Chem., 30(3), 447-457 (2006)
David E Stephens et al.
Organic & biomolecular chemistry, 12(32), 6190-6199 (2014-07-06)
The scope and mechanistic implications of the direct transformation of heterocyclic N-oxides to 2-trifluoromethyl-, and related perfluoroalkyl- and perfluoroaryl-substituted N-heterocycles has been studied. The reaction is effected by perfluoroalkyl- and perfluorophenyltrimethylsilane in the presence of strong base. In situ displacement
相關內容
The major research interests of Prof. Jinbo Hu's lab include the development of new fluorination reagents and reactions, especially the difluoromethylation, difluoromethylenation, and monofluoromethylation methods.
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務