全部照片(1)
About This Item
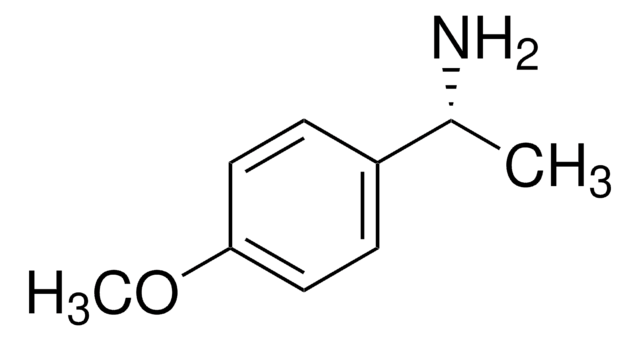
線性公式:
C6H11CH(CH3)NH2
CAS號碼:
分子量::
127.23
Beilstein:
2935069
MDL號碼:
分類程式碼代碼:
12352116
PubChem物質ID:
NACRES:
NA.22
推薦產品
等級
produced by BASF
化驗
≥98.5% (GC)
99%
形狀
liquid
光學純度
enantiomeric excess: ≥98.5%
折射率
n20/D 1.4614 (lit.)
bp
60 °C/12 mmHg (lit.)
密度
0.856 g/mL at 25 °C (lit.)
SMILES 字串
C[C@H](N)C1CCCCC1
InChI
1S/C8H17N/c1-7(9)8-5-3-2-4-6-8/h7-8H,2-6,9H2,1H3/t7-/m0/s1
InChI 密鑰
XBWOPGDJMAJJDG-ZETCQYMHSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
(S)-(+)-1-Cyclohexylethylamine is a chiral amine.
應用
(S)-(+)-1-Cyclohexylethylamine may be used in the synthesis of (S)-(-)-ferrocenylimine by reacting with ferrocenecarboxaldehyde.
法律資訊
ChiPros is a registered trademark of BASF SE
訊號詞
Danger
危險聲明
危險分類
Aquatic Chronic 2 - Flam. Liq. 3 - Skin Corr. 1B
儲存類別代碼
3 - Flammable liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
125.6 °F - closed cup
閃點(°C)
52 °C - closed cup
分析證明 (COA)
輸入產品批次/批號來搜索 分析證明 (COA)。在產品’s標籤上找到批次和批號,寫有 ‘Lot’或‘Batch’.。
Isoindolinones via a room temperature palladium nanoparticle-catalysed 3-component cyclative carbonylation?amination cascade
Grigg, Ronald, et al.
Tetrahedron, 44.37, 6979-6982 (2003)
Yee Voan Teo et al.
Cell reports, 27(4), 997-1007 (2019-04-25)
Oncogene-induced senescence (OIS) is a tumor suppressive response to oncogene activation that can be transmitted to neighboring cells through secreted factors of the senescence-associated secretory phenotype (SASP). Currently, primary and secondary senescent cells are not considered functionally distinct endpoints. Using
Supramolecular chirogenesis in bis (zinc porphyrin): an absolute configuration probe highly sensitive to guest structure
Borovkov, Victor V., Juha M. Lintuluoto, and Yoshihisa Inoue
Organic Letters, 2.11, 1565-1568 (2000)
Expedient synthesis and design strategies for new peptoid construction
Gorske, Benjamin C., et al.
Organic Letters, 7.8, 1521-1524 (2005)
Synthesis of optically-active planar chiral derivatives of ferrocene. Crystal structures of alkyne insertion products.
Zhao G, et al.
Tetrahedron Asymmetry, 9(13), 2253-2257 (1998)
文章
Chiral amines play an important role in stereoselective organic synthesis. They are used directly as resolving agents, building blocks, or chiral auxiliaries.
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務