推薦產品
化驗
97%
形狀
solid
mp
36-40 °C (lit.)
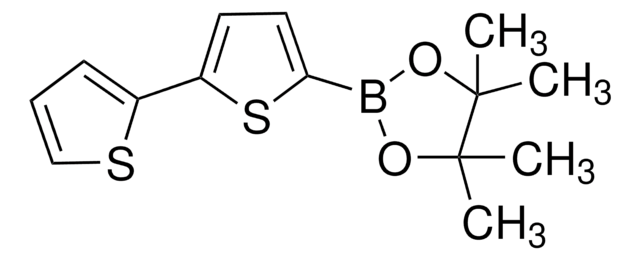
SMILES 字串
CCCCCCc1ccc(s1)-c2ccc(s2)B3OC(C)(C)C(C)(C)O3
InChI
1S/C20H29BO2S2/c1-6-7-8-9-10-15-11-12-16(24-15)17-13-14-18(25-17)21-22-19(2,3)20(4,5)23-21/h11-14H,6-10H2,1-5H3
InChI 密鑰
XTTRNSNHDCYSEL-UHFFFAOYSA-N
應用
Reagent use for
Reagent used in Preparation of
- Suzuki-Miyaura cross-coupling reactions and shape-shifting in contorted dibenzotetrathienocoronenes
- Oligothiophene self-assembly induction into fibers with tunable shape and function
- Stille coupling and p-conjugated packing structure and hole mobility of bithiophene-bithiazole copolymers with alkyl-thiophene side chains
Reagent used in Preparation of
- Solution-processed ambipolar field-effect transistor
- Light harvesting small molecules for use in solution-processed small molecule bulk heterojunction solar cell devices
- Light-emitting diode (OLED) materials
- Unsymmetric substituted benzothiadiazole-containing vinyl monomers for RAFT polymerization
- Pd-catalyzed condensations and synthesis of isoindigo-based oligothiophenes for molecuar bulk heterojunction solar cells
- Thiophene-benzothiadiazole based donor-acceptor-donor materials
分析證明 (COA)
輸入產品批次/批號來搜索 分析證明 (COA)。在產品’s標籤上找到批次和批號,寫有 ‘Lot’或‘Batch’.。
Synthesis and light-emitting properties of solution-processable oligothiophene derivatives containing triazole moiety
Kim, B.; et al.
Materials Science Forum, 663-665 (2011)
Shape-shifting in contorted dibenzotetrathienocoronenes
Chiu, C-Y.; et al.
Chemical Science, 2, 1480-1486 (2011)
Synthesis, characterization and comparative study of thiophene-benzothiadiazole based donor-acceptor-donor (D-A-D) materials
Sonar, P.; et al.
Journal of Materials Chemistry, 19, 3228-3237 (2009)
Bithiophene-bithiazole alternating copolymers with thiophene side chains: Synthesis by organometallic polycondensation and chemical properties of the copolymers
Yamamoto, T.; et al.
Journal of Polymer Science Part A: Polymer Chemistry, 49, 1508-1512 (2011)
Benzothiadiazole-Containing Pendant Polymers Prepared by RAFT and Their Electro-Optical Properties
Haussler, M.; et al.
Macromolecules, 43, 7101-7110 (2010)
文章
Oligothiophenes are important organic electronic materials which can be produced using synthetic intermediates and Suzuki coupling.
Suzukii-Miyaura 交叉偶合反應廣泛應用於有機化學、聚合物科學和製藥工業。
Suzuki-Miyaura cross-coupling reaction is extensively used in organic chemistry, polymer science, and pharmaceutical industries.
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務