推薦產品
化驗
97%
形狀
solid
mp
82-86 °C (lit.)
儲存溫度
2-8°C
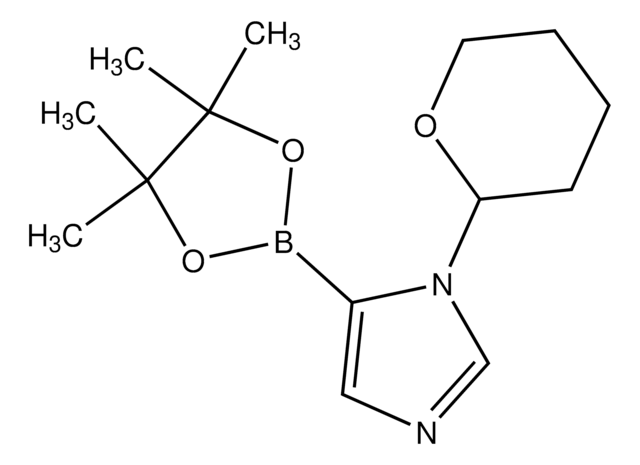
SMILES 字串
CC(C)(C)OC(=O)n1cc(cn1)B2OC(C)(C)C(C)(C)O2
InChI
1S/C14H23BN2O4/c1-12(2,3)19-11(18)17-9-10(8-16-17)15-20-13(4,5)14(6,7)21-15/h8-9H,1-7H3
InChI 密鑰
IPISOFJLWYBCAV-UHFFFAOYSA-N
應用
Reagent used for
Reagent used in Preparation of
- Suzuki Coupling
- Copper-catalyzed azidation
Reagent used in Preparation of
- Selective quinazolinyl-phenol inhibitors of CHK1 as potential antitumors and radioprotectants
- Stereoselective synthesis of selective Cathepsin inhibitors
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
分析證明 (COA)
輸入產品批次/批號來搜索 分析證明 (COA)。在產品’s標籤上找到批次和批號,寫有 ‘Lot’或‘Batch’.。
客戶也查看了
John J Caldwell et al.
Journal of medicinal chemistry, 54(2), 580-590 (2010-12-29)
Structure-based design was applied to the optimization of a series of 2-(quinazolin-2-yl)phenols to generate potent and selective ATP-competitive inhibitors of the DNA damage response signaling enzyme checkpoint kinase 2 (CHK2). Structure-activity relationships for multiple substituent positions were optimized separately and
Copper(II)-catalyzed conversion of aryl/heteroaryl boronic acids, boronates, and trifluoroborates into the corresponding azides: substrate scope and limitations
K. D. Grimes, et al.,
Synthesis, 9, 1441-1448 (2010)
Paul A Bethel et al.
Bioorganic & medicinal chemistry letters, 19(16), 4622-4625 (2009-07-21)
A number of molecular recognition features have been exploited in structure-based design of selective Cathepsin inhibitors.
文章
Suzukii-Miyaura 交叉偶合反應廣泛應用於有機化學、聚合物科學和製藥工業。
Suzuki-Miyaura cross-coupling reaction is extensively used in organic chemistry, polymer science, and pharmaceutical industries.
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務




![[1,1′-双(二苯基膦)二茂铁]二氯化钯(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)
![[1,1′-双(二苯基膦)二茂铁]二氯化钯(II)二氯甲烷络合物](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)