推薦產品
化驗
98%
mp
114-116 °C (lit.)
SMILES 字串
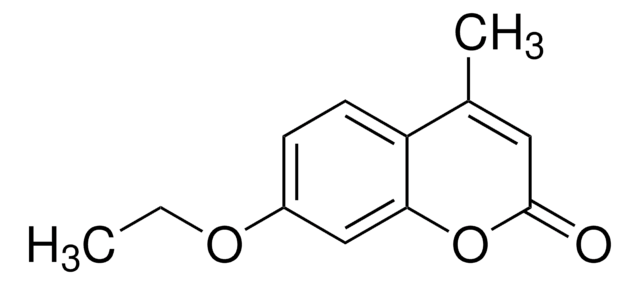
CCOc1ccc2C(C)=CC(=O)Oc2c1
InChI
1S/C12H12O3/c1-3-14-9-4-5-10-8(2)6-12(13)15-11(10)7-9/h4-7H,3H2,1-2H3
InChI 密鑰
NKRISXMDKXBVRJ-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
7-Ethoxy-4-methylcoumarin (EtOMC), also known as ethyl 4-methylumbelliferyl ether, is a coumarin derivative. Its standard molar energy of combustion is ?5888.0 ± 3.2kJ·mol?1 and standard molar enthalpy of formation in the crystalline phase is ?545.4 ± 3.6kJ·mol?1.EtOMC can be prepared by reacting methyl acetoacetate with 3-ethoxyphenol in the presence of boron trifluoride dihydrate.The fluorescence quenching of EtOMC is more effective in the presence 4-hydroxy-TEMPO (4-hydroxy-2,2,6,6-tetramethylpiperidin-1-oxyl).
應用
7-Ethoxy-4-methylcoumarin may be used in the assay of 4-chlormethyl-7-ethoxycoumarin O-deethylase activity.
儲存類別代碼
13 - Non Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
Analysis of fluorescence quenching of coumarin derivatives by 4-hydroxy-TEMPO in aqueous solution.
Zamojc K, et al.
Journal of Fluorescence, 24(3), 713-718 (2014)
Standard molar enthalpies of formation in the crystalline phase of 7-hydroxy-4-methylcoumarin, 7-ethoxy-4-methylcoumarin, and 6-methoxy-4-methylcoumarin.
Amador P, et al.
The Journal of Chemical Thermodynamics, 43(9), 1414-1416 (2011)
U D Kuhn et al.
Experimental and toxicologic pathology : official journal of the Gesellschaft fur Toxikologische Pathologie, 50(4-6), 491-496 (1998-10-23)
7-Ethoxycoumarin (EC) is widely used as a model substrate for monooxygenase function, its O-deethylation representing cytochrome P450 (P450) activity mainly of 1A but also of 2B isoforms. Reports on investigations of its own capacity to induce or suppress P450 activities
Pechmann reaction promoted by boron trifluoride dihydrate.
Stoyanov E and Mezger J.
Molecules (Basel), 10(7), 762-766 (2005)
S Ekins et al.
The Journal of pharmacology and experimental therapeutics, 286(3), 1253-1259 (1998-09-11)
Previous studies in this laboratory have determined the lack of specificity of several antibody and substrate probes of CYP2B6. The goals of the current study were to examine the expression of CYP2B6 in a bank of human liver microsome (HLM)
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務