全部照片(1)
About This Item
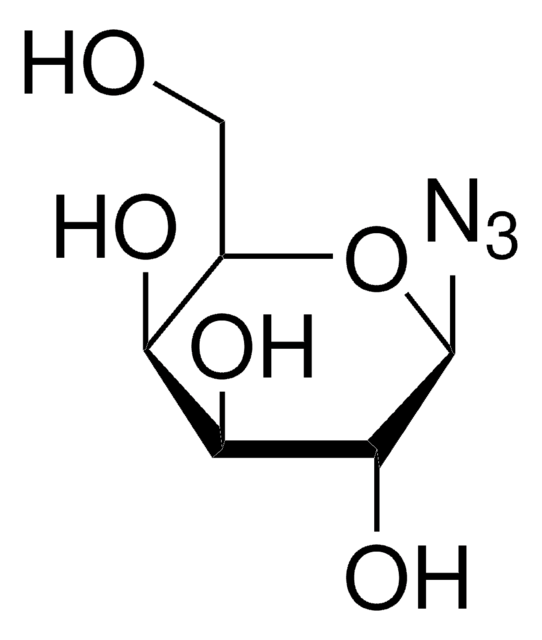
經驗公式(希爾表示法):
C14H19N3O9
CAS號碼:
分子量::
373.32
MDL號碼:
分類程式碼代碼:
12352200
PubChem物質ID:
NACRES:
NA.22
推薦產品
形狀
solid
光學活性
[α]/D -29°, c = 1% in H2O
[α]/D -30°, c = 1% in chloroform
反應適用性
reaction type: click chemistry
mp
127-131 °C (lit.)
SMILES 字串
CC(=O)OC[C@H]1O[C@@H](N=[N+]=[N-])[C@H](OC(C)=O)[C@@H](OC(C)=O)[C@H]1OC(C)=O
InChI
1S/C14H19N3O9/c1-6(18)22-5-10-11(23-7(2)19)12(24-8(3)20)13(25-9(4)21)14(26-10)16-17-15/h10-14H,5H2,1-4H3/t10-,11+,12+,13-,14-/m1/s1
InChI 密鑰
NHNYHKRWHCWHAJ-MBJXGIAVSA-N
應用
1-Azido-1-deoxy-β-D-glucopyranoside tetraacetate can be used as a reactant to synthesize:
- 1,2,3-Triazole-boron dipyrromethenes (BODIPYs) containing glucose groups via Cu(I)-catalyzed azide–alkyne ″click″ cycloaddition reaction conditions.
- 1-(β-D-glycosyl)-5-benzenesulfonamide-1,2,3-triazole derivatives by ruthenium-catalyzed azide-alkyne cycloaddition reactions.
- 2,3,4,6-Tetra-O-acetyl-β-D-glucopyranosylamine by palladium catalyzed hydrogenation reaction.
- Glycoside annulated dihydropyrimidinone derivatives by one-pot five-component condensation reaction with tert-butyl β-ketoester, arylaldehyde, urea and propargyl alcohol.
Reactant for:
- Synthesis of Protein Tyrosine Phosphatase 1B inhibitor
- Synthesis of glycoconjugate carbonic anhydrase inhibitors by ruthenium-catalyzed azide-alkyne 1,3-dipolar cycloaddition
- Synthesis of variously coupled conjugates of D-glucose via click chemistry for inhibition of glycogen phosphorylase
- Hydrogenation reactions
- Preparation of posttranslationally modified peptides efficiently mimicking neoantigens in relation to autoimmune disease
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
Hosahalli P Hemantha et al.
Organic & biomolecular chemistry, 9(8), 2597-2601 (2011-03-09)
Palladium nanoparticles supported over poly(vinyl)chloride matrix (PVC-Pd(0)) are prepared through an efficient and inexpensive protocol. The catalyst has been characterized by XRD, SEM and TEM and its utility for the reduction of a range of functional groups as well as
A facile one-pot five-component synthesis of glycoside annulated dihydropyrimidinone derivatives with 1, 2, 3-triazol linkage via transesterification/Biginelli/click reactions in aqueous medium
Rao GB Dharma, et al.
Tetrahedron Letters, 55(1) (2014)
Syntheses of 1, 2, 3-triazole-BODIPYs bearing up to three carbohydrate units
Nguyen AL, et al.
New. J. Chem., 42(10) (2018)
Adam J Salmon et al.
Bioorganic & medicinal chemistry letters, 21(20), 6058-6061 (2011-09-10)
Carbonic anhydrase IX (CA IX) is a recently validated target for the development of new cancer therapies. In this Letter we describe the synthesis and CA inhibition of a novel series of carbohydrate-based 1,5-disubstituted-1,2,3-triazole benzenesulfonamides. The key step of our
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務