全部照片(2)
About This Item
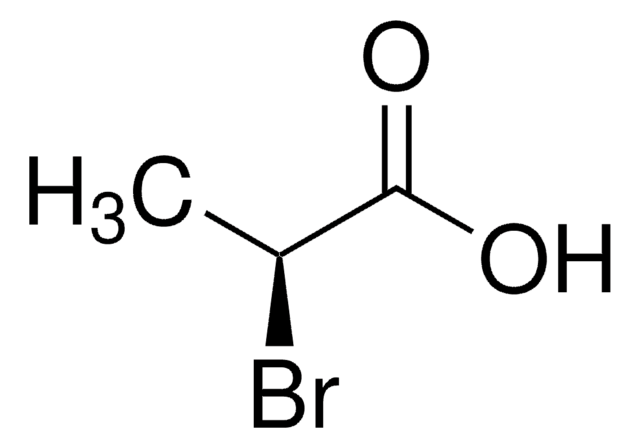
線性公式:
(CH3)2CHCH(Br)CO2H
CAS號碼:
分子量::
181.03
MDL號碼:
分類程式碼代碼:
12352101
PubChem物質ID:
NACRES:
NA.22
推薦產品
化驗
96%
光學活性
[α]22/D +21°, c = 37 in benzene
bp
90-100 °C/0.5 mmHg (lit.)
mp
35-40 °C (lit.)
SMILES 字串
CC(C)[C@@H](Br)C(O)=O
InChI
1S/C5H9BrO2/c1-3(2)4(6)5(7)8/h3-4H,1-2H3,(H,7,8)/t4-/m1/s1
InChI 密鑰
UEBARDWJXBGYEJ-SCSAIBSYSA-N
應用
(R)-(+)-2-Bromo-3-methylbutyric acid can be used:
- As an anionic counterpart of morpholinium based chiral ionic liquids applicable in the Heck arylation reaction of 2,3-dihydrofuran.
- As a starting material in the synthesis of fructosyl peptide oxidase inhibitors.
訊號詞
Danger
危險分類
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Dam. 1 - Skin Corr. 1B
儲存類別代碼
8A - Combustible corrosive hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
235.4 °F - closed cup
閃點(°C)
113 °C - closed cup
個人防護裝備
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
分析證明 (COA)
輸入產品批次/批號來搜索 分析證明 (COA)。在產品’s標籤上找到批次和批號,寫有 ‘Lot’或‘Batch’.。
Effect of chiral ionic liquids on palladium-catalyzed Heck arylation of 2, 3-dihydrofuran
Roszak R, et al.
Applied Catalysis A: General, 409, 148-155 (2011)
Synthesis and inhibitory activity of substrate-analog fructosyl peptide oxidase inhibitors
Watanabe B, et al.
Bioorganic & Medicinal Chemistry Letters, 25(18), 3910-3913 (2015)
J M Te Koppele et al.
The Biochemical journal, 252(1), 137-142 (1988-05-15)
The stereoselectivity of purified rat GSH transferases towards alpha-bromoisovaleric acid (BI) and its amide derivative alpha-bromoisovalerylurea (BIU) was investigated. GSH transferase 2-2 was the only enzyme to catalyse the conjugation of BI and was selective for the (S)-enantiomer. The conjugation
M Polhuijs et al.
Biochemical pharmacology, 44(7), 1249-1253 (1992-10-06)
Glutathione (GSH) conjugation of the separate enantiomers of five 2-bromocarboxylic acids and some of their urea derivatives by rat liver GSH transferases (GSTs) was studied. The liver cytosolic fraction conjugated all compounds, except for (R)-2-bromoisovaleric acid, with a variable degree
M Polhuijs et al.
Biochemical pharmacology, 38(22), 3957-3962 (1989-11-15)
The glutathione (GSH) conjugation of (R)-and (S)-alpha-bromoisovaleric acid (BI) in the rat in vivo, and its stereoselectivity, have been characterized. After administration of racemic [1-14C]BI two radioactive metabolites were found in bile: only one of the possible diastereomeric BI-GSH conjugates
Chromatograms
application for HPLC我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務