全部照片(2)
About This Item
線性公式:
(CH3)3CSi(CH3)2CN
CAS號碼:
分子量::
141.29
Beilstein:
2234709
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.22
推薦產品
化驗
97%
mp
76-79 °C (lit.)
SMILES 字串
CC(C)(C)[Si](C)(C)C#N
InChI
1S/C7H15NSi/c1-7(2,3)9(4,5)6-8/h1-5H3
InChI 密鑰
CWAKIXKDPQTVTA-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
tert-Butyldimethylsilyl cyanide (TBDMSCN) is a bulkier trialkylsilylcyanide. It participates in the cyanosilylation of enantiopure 4-oxoazetidine-2-carbaldehydes. Addition of TBDMSCN to sterically hindered ketones in the presence of Lewis acid or base catalyst has been studied. ZnI2-catalyzed addition of TBDSCN to 2,2-dimethylcyclohexanone, 2,2,6-trimethylcyclohexanone and 2,2,6,6-tetramethylcyclohexanone affords protected cyanohydrins.
應用
tert-Butyldimethylsilyl cyanide may be used as reagent for the formation of β-isonitrile alcohols via epoxides.
訊號詞
Danger
危險分類
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral
儲存類別代碼
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
水污染物質分類(WGK)
WGK 3
個人防護裝備
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
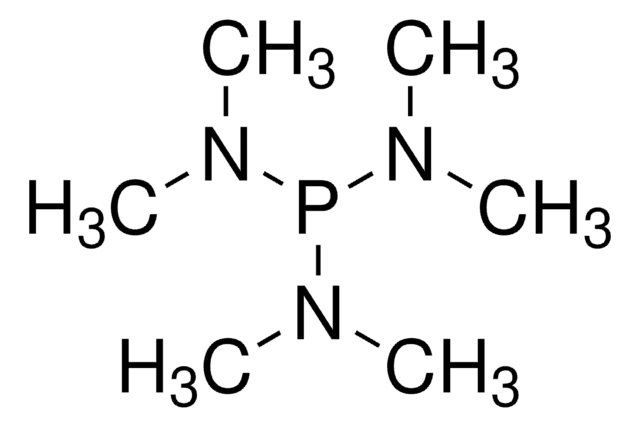
P(RNCH2CH2)N: efficient catalysts for the cyanosilylation of aldehydes and ketones.
Fetterly BM and Verkade JG.
Tetrahedron Letters, 46(46), 8061-8066 (2005)
The Journal of Organic Chemistry, 51, 5010-5010 (1986)
Addition of tert-butyldimethyl-or tert-butyldiphenylsilyl cyanide to hindered ketones.
Golinski M, et al.
The Journal of Organic Chemistry, 58(1), 159-164 (1993)
Benito Alcaide et al.
The Journal of organic chemistry, 72(21), 7980-7991 (2007-09-18)
The cyanosilylation of enantiopure 4-oxoazetidine-2-carbaldehydes with tert-butyldimethylsilyl cyanide was promoted by either molecular sieves or catalytic amount of sodium carbonate to give O-silylated beta-lactam cyanohydrins with good yield and diastereoselectivity. In contrast, Lewis acids did not effectively promote the cyanosilylation
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務