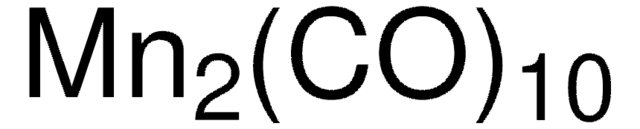推薦產品
蒸汽壓力
2.15 psi ( 20 °C)
化驗
96%
折射率
n20/D 1.262 (lit.)
bp
68-69 °C/743 mmHg (lit.)
密度
1.736 g/mL at 25 °C (lit.)
SMILES 字串
FC(F)(F)C(F)(F)N(C(F)(F)C(F)(F)F)C(F)(F)C(F)(F)F
InChI
1S/C6F15N/c7-1(8,9)4(16,17)22(5(18,19)2(10,11)12)6(20,21)3(13,14)15
InChI 密鑰
CBEFDCMSEZEGCX-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
Pentadecafluorotriethylamine (Perfluorotriethylamine, Tris(pentafluoroethyl)amine) is a non-polar organo fluorine compound. Its various physical parameters have been reported. It is an efficient substitute of reaction medium for the Lewis acid mediated reactions. Pyrolysis of perfluorotriethylamine was studied to investigate its thermal behavior in firefighting. Perfluorinated N-ethylpyrrolidine has been identified as the major decomposition product by GC-MS analysis. Production of long-lived microbubbles (50-100μm) in perfluorotriethylamine has been studied.
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
客戶也查看了
Thermal decomposition of halon alternatives.
Yamamoto T, et al.
Chemosphere, 35(3), 643-654 (1997)
The behavior of bubbles in perfluorotriethylamine under the effect of strong electric fields.
Korobeinikov SM, et al.
High Temperature, 39(6), 821-825 (2001)
Organic reactions without an organic medium. Utilization of perfluorotriethylamine as a reaction medium.
Nakano H and Kitazume T.
Green Chemistry, 1(1), 21-22 (1999)
An efficient high-yield synthesis for perfluorinated tertiary alkyl amines.
Felling KW and Lagow RJ.
Journal of Fluorine Chemistry, 123(2), 233-236 (2003)
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務