推薦產品
化驗
97%
mp
149-152 °C (lit.)
溶解度
methanol: soluble 25 mg/mL, clear, colorless to yellow
SMILES 字串
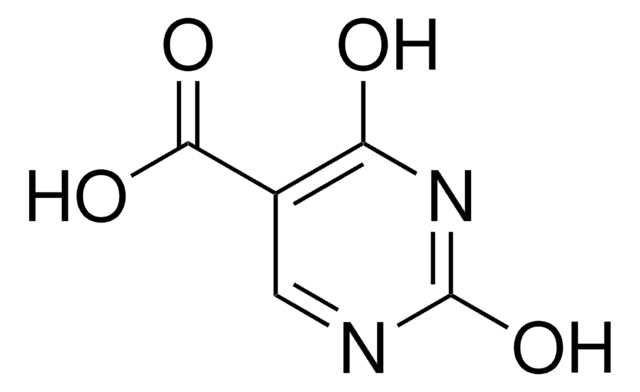
COc1cc(N)nc(OC)n1
InChI
1S/C6H9N3O2/c1-10-5-3-4(7)8-6(9-5)11-2/h3H,1-2H3,(H2,7,8,9)
InChI 密鑰
LNTJJKHTAZFVJJ-UHFFFAOYSA-N
一般說明
4-Amino-2,6-dimethoxypyrimidine is methoxy substituted 4-aminopyrimidine. Molecules of 4-amino-2,6-dimethoxypyrimidine are linked by an N-H.O hydrogen bond and an N-H.N hydrogen bond, forming sheets containing centrosymmetric rings. Photocatalytic degradation of 4-amino-2,6-dimethoxypyrimidine on TiO2 has been reported. Mass spectra of 4-amino-2,6-dimethoxypyrimidine has been studied.
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
分析證明 (COA)
輸入產品批次/批號來搜索 分析證明 (COA)。在產品’s標籤上找到批次和批號,寫有 ‘Lot’或‘Batch’.。
Christopher Glidewell et al.
Acta crystallographica. Section C, Crystal structure communications, 59(Pt 4), O202-O204 (2003-04-12)
Molecules of the title compound, C(6)H(9)N(3)O(2), are linked by an N-H.O hydrogen bond [H.O = 2.29 A, N.O = 3.169 (2) A and N-H.O = 173 degrees ] and an N-H.N hydrogen bond [H.N = 2.12 A, N.N = 2.999
Mass spectra of methoxy-substituted 4-aminopyrimidines.
Khmel'nitskii RA, et al.
Chemistry of Heterocyclic Compounds, 10(1), 113-116 (1974)
Photocatalytic transformations of aminopyrimidines on TiO< sub> 2</sub> in aqueous solution.
Calza P, et al.
Applied Catalysis. B, Environmental, 52(4), 267-274 (2004)
Monica Olivella et al.
Archiv der Pharmazie, 348(1), 68-80 (2014-11-22)
New nitrosopyrimidines were synthesized and evaluated as potential antibacterial agents. Different compounds structurally related with 4,6-bis(alkyl or arylamino)-5-nitrosopyrimidines were evaluated. Some of these nitrosopyrimidines displayed significant antibacterial activity against human pathogenic bacteria. Among them compounds 1c, 2a-c, and 9a-c exhibited
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務