推薦產品
化驗
96%
形狀
solid
mp
270-273 °C (lit.)
SMILES 字串
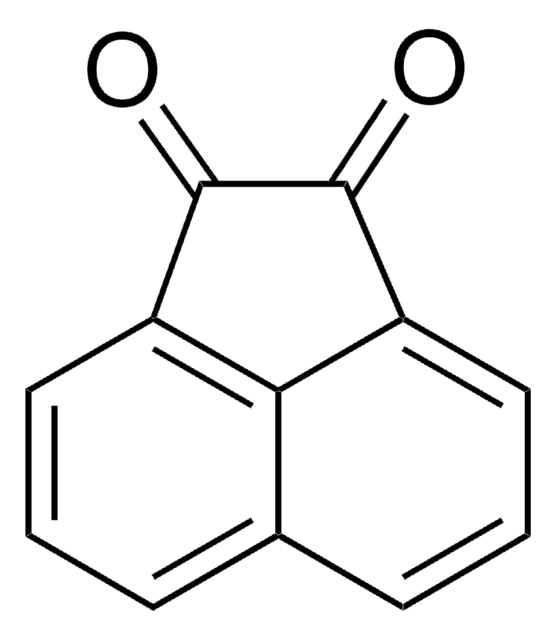
O=C1C(=O)c2c3ccccc3cc4cccc1c24
InChI
1S/C16H8O2/c17-15-12-7-3-5-10-8-9-4-1-2-6-11(9)14(13(10)12)16(15)18/h1-8H
InChI 密鑰
YAIBDWAANBTYIA-UHFFFAOYSA-N
一般說明
醋蒽醌是一种环状 α-二酮。 在富勒烯C(60)存在下,它与六乙基亚磷酸胺反应,生成亚甲基富勒烯衍生物。 醋蒽醌与一系列芳烃的羟烷基化反应已有报道。
應用
醋蒽醌被用于合成spiro-tricyclic porphodimethene。
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
分析證明 (COA)
輸入產品批次/批號來搜索 分析證明 (COA)。在產品’s標籤上找到批次和批號,寫有 ‘Lot’或‘Batch’.。
M Harmjanz et al.
Organic letters, 3(15), 2281-2284 (2001-07-21)
[structure: see text] Acid-catalyzed [2 + 2] condensation reactions of polycyclic aromatic vicinal diketones including aceanthrenequinone, phenathrenequinone, and pyrene-4,5-dione with 5-mesityldipyrromethanes are outlined, and this methodology provides a flexible entry to spiro-tricyclic porphodimethenes. The porphodimethene products have been fully characterized
Irina P Romanova et al.
The Journal of organic chemistry, 76(8), 2548-2557 (2011-03-12)
The reactions of such cyclic α-diketones as acenaphthenequinone, aceanthrenequinone, and N-alkylisatins, with hexaethyltriaminophosphine in the presence of the fullerene C(60), lead to the formation of methanofullerene derivatives under mild conditions. This process proceeds via deoxygenation of the dicarbonyl compound by
Douglas A Klumpp et al.
Applied catalysis. A, General, 336(1-2), 128-132 (2008-03-01)
The hydroxyalkylation reactions of aceanthrenequinone (6) and acenapthenequinone (7) with a series of arenes have been studied. In reactions with the Brønsted superacid CF(3)SO(3)H (triflic acid), the condensation products are formed in good yields (58-99%, 10 examples) with high regioselectivity.
Janice L Hyatt et al.
Journal of medicinal chemistry, 50(23), 5727-5734 (2007-10-19)
Carboxylesterases (CE) are ubiquitous enzymes responsible for the detoxification of xenobiotics, including numerous clinically used drugs. Therefore, the selective inhibition of these proteins may prove useful in modulating drug half-life and bioavailability. Recently, we identified 1,2-diones as potent inhibitors of
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務