全部照片(1)
About This Item
經驗公式(希爾表示法):
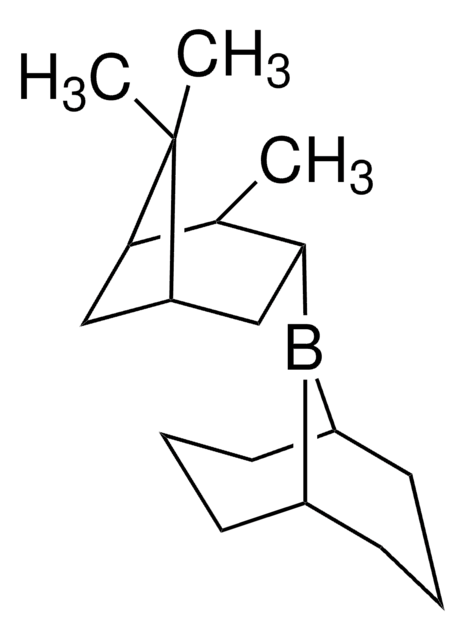
C18H31B
CAS號碼:
分子量::
258.25
MDL號碼:
分類程式碼代碼:
12352000
PubChem物質ID:
NACRES:
NA.22
推薦產品
形狀
liquid
光學活性
[α]22/D +1.2°, neat
濃度
0.5 M in THF
密度
0.897 g/mL at 25 °C
SMILES 字串
C[C@@H]1[C@H](C[C@H]2C[C@@H]1C2(C)C)B3[C@@H]4CCC[C@H]3CCC4
InChI
1S/C18H31B/c1-12-16-10-13(18(16,2)3)11-17(12)19-14-6-4-7-15(19)9-5-8-14/h12-17H,4-11H2,1-3H3/t12-,13+,14-,15+,16-,17-/m0/s1
InChI 密鑰
VCDGSBJCRYTLNU-NEXGVSGLSA-N
一般說明
S-Alpine-Borane® is a chiral reducing agent, synthesized from (-)-α-pinene via hydroboration.
應用
R- 和 S-Alpine-Boranes 用于醛和前手性酮的不对称还原反应。
S-Alpine-Borane® solution may be used to prepare:
- 5(S)-methyl 5-hydroxy-8-(tetrahydropyran-2-yloxy)-6-octynoate, an intermediate for the stereocontrolled total synthesis of leukotriene B4.
- (+)-(S)-4-hydroxy-6-phenyl-5-hexynyl 2,2,4,4-tetramethyl-1,3-oxazolidine-3-carboxylate
- (R)-1-(tert-Butyldiphenylsilyloxy)pent-3-yn-2-ol, an intermediate for the preparation of (S)-4,5-dihydroxypentane-2,3-dione (DPD).
法律資訊
Alpine-Borane is a registered trademark of Merck KGaA, Darmstadt, Germany
訊號詞
Danger
危險分類
Acute Tox. 4 Oral - Carc. 2 - Eye Irrit. 2 - Flam. Liq. 2 - Pyr. Liq. 1 - STOT SE 3
標靶器官
Respiratory system
安全危害
儲存類別代碼
4.2 - Pyrophoric and self-heating hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
1.4 °F - closed cup
閃點(°C)
-17 °C - closed cup
個人防護裝備
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
分析證明 (COA)
輸入產品批次/批號來搜索 分析證明 (COA)。在產品’s標籤上找到批次和批號,寫有 ‘Lot’或‘Batch’.。
(-)-Sparteine-Mediated Stereoselective Intramolecular Carbolithiation of 4-Substituted 5-Hexynyl Carbamates. Synthesis of Enantiopure 1, 3-Difunctionalized Alkylidene Cyclopentanes.
Oestreich M, et al.
The Journal of Organic Chemistry, 64(23), 8616-8626 (1999)
An efficient synthesis of the precursor of AI-2, the signalling molecule for inter-species quorum sensing.
Ascenso OS, et al.
Bioorganic & Medicinal Chemistry, 19(3), 1236-1241 (2011)
Total synthesis of leukotriene B4.
Kerdesky FAJ, et al.
The Journal of Organic Chemistry, 58(13), 3516-3520 (1993)
Tetrahedron Asymmetry, 5, 1061-1061 (1994)
Aldrichimica Acta, 15, 68-68 (1982)
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務