推薦產品
蒸汽壓力
0.01 mmHg ( 25 °C)
化驗
99%
形狀
solid
bp
260-262 °C (lit.)
mp
39-42 °C (lit.)
溶解度
alcohol: freely soluble
benzene: freely soluble
chloroform: freely soluble
diethyl ether: freely soluble
petroleum ether: very slightly soluble
water: very slightly soluble
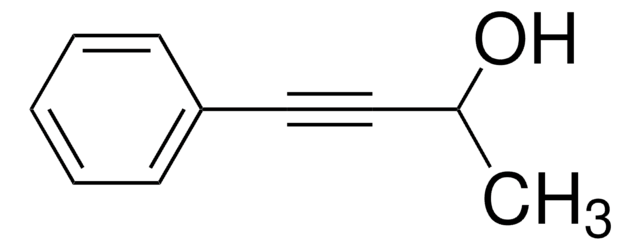
SMILES 字串
[H]\C(=C(\[H])c1ccccc1)C(C)=O
InChI
1S/C10H10O/c1-9(11)7-8-10-5-3-2-4-6-10/h2-8H,1H3/b8-7+
InChI 密鑰
BWHOZHOGCMHOBV-BQYQJAHWSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
反式 -4-苯基-3-丁烯-2-酮是谷胱甘肽转移酶的底物 。它与甲基 - 和苄基胍反应生成芳香族N2-取代的2-嘧啶胺。
訊號詞
Warning
危險聲明
危險分類
Skin Irrit. 2 - Skin Sens. 1
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 2
閃點(°F)
253.4 °F - closed cup
閃點(°C)
123 °C - closed cup
個人防護裝備
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
分析證明 (COA)
輸入產品批次/批號來搜索 分析證明 (COA)。在產品’s標籤上找到批次和批號,寫有 ‘Lot’或‘Batch’.。
客戶也查看了
Dihydropyrimidines and related structures. I. N2-substituted 2-pyrimidinamines and dihydro-2-pyrimidinamines by reaction of phenylbutenones and monosubstituted guanidines.
Winfried W and Schermanz K.
Journal of Heterocyclic Chemistry, 21(1), 65-69 (1984)
Glutathione S-transferases. The first enzymatic step in mercapturic acid formation.
W H Habig et al.
The Journal of biological chemistry, 249(22), 7130-7139 (1974-11-25)
Chisako Yamagami et al.
Bioorganic & medicinal chemistry letters, 12(17), 2281-2285 (2002-08-06)
The inhibitory effect (IC(50)) of the title compounds on UV-induced mutagenesis in Escherichia coli WP2uvrA was analyzed quantitatively by using various quantum chemical descriptors and also by the CoMFA method: both approaches provided results of similar quality. The activity was
Bin Cao et al.
Organic & biomolecular chemistry, 10(6), 1239-1245 (2011-12-20)
An efficient and easy procedure to synthesize the pyridinyl analogues of dibenzylideneacetone (pyr-dba) was developed by the condensation of substituted nicotinaldehyde and acetone in the presence of K(2)CO(3) in toluene-EtOH-H(2)O solvent system. Structurally diverse pyr-dba, including quinolinyl dba, can be
Jiyong Zhang et al.
The Journal of organic chemistry, 71(7), 2918-2921 (2006-03-25)
The first efficient asymmetric synthesis of obolactone 1 has been accomplished in 11 steps and with a 15% overall yield in which Brown's enantioselective allylation reactions and ring-closing metathesis reaction are key steps.
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務