全部照片(1)
About This Item
經驗公式(希爾表示法):
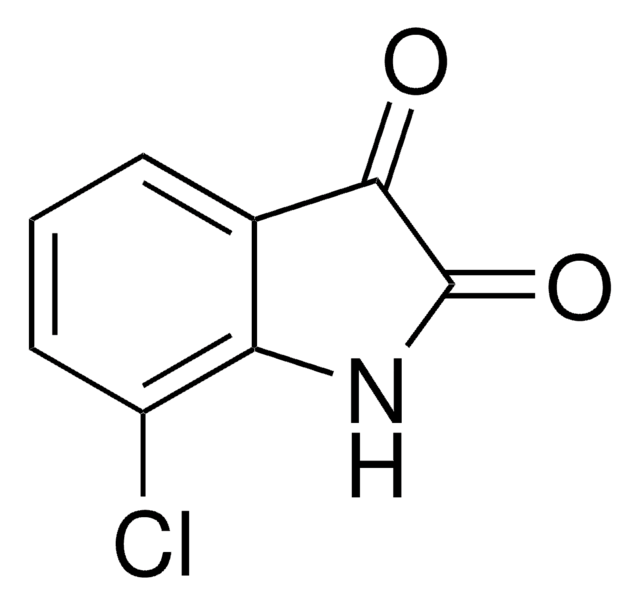
C8H4ClNO2
CAS號碼:
分子量::
181.58
EC號碼:
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.22
推薦產品
化驗
97%
mp
254-258 °C (lit.)
SMILES 字串
Clc1ccc2NC(=O)C(=O)c2c1
InChI
1S/C8H4ClNO2/c9-4-1-2-6-5(3-4)7(11)8(12)10-6/h1-3H,(H,10,11,12)
InChI 密鑰
XHDJYQWGFIBCEP-UHFFFAOYSA-N
一般說明
5-Chloroisatin reacts with substituted 4-amino-4,5-dihydro-1H-1,2,4-triazole-5-ones to yield Schiff bases.
應用
5-Chloroisatin was used in synthesis of Schiff base by reaction with 2-methyl-4-nitroaniline.
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
客戶也查看了
Synthesis, Characterization and Biological Activities of a New 5-Chloroisatin Schiff Base and its Metal Complexes.
RAO R, et al.
Chemical Science Transactions, 2(3), 1063-1069 (2013)
Olcay Bekircan et al.
Molecules (Basel, Switzerland), 13(9), 2126-2135 (2008-10-03)
Ethyl imidate hydrochlorides 1 were prepared by passing HCl gas through solutions of substituted benzyl cyanides and absolute ethanol. Ethoxycarbonylhydrazones 2 were synthesized from the reaction of compounds 1 with ethyl carbazate. Treatment of 2 with hydrazine hydrate leads to
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務