08751
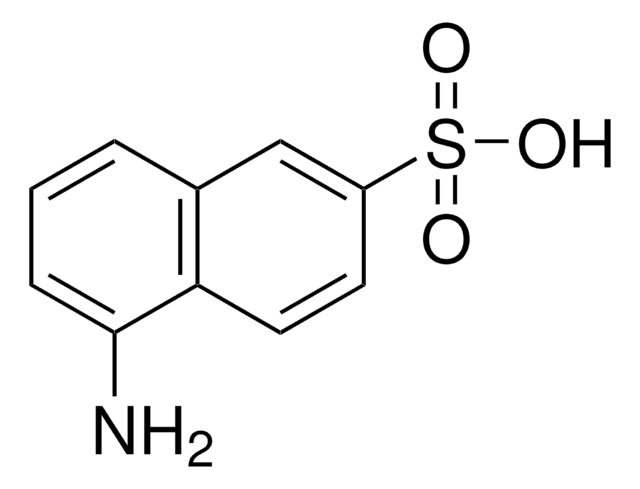
4-Amino-3-hydroxy-1-naphthalenesulfonic acid
for spectrophotometric det. of Si, ≥90.0% (CHN)
Synonym(s):
1-Amino-2-naphthol-4-sulfonic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
H2NC10H5(OH)SO3H
CAS Number:
Molecular Weight:
239.25
Beilstein:
2697469
EC Number:
MDL number:
UNSPSC Code:
41116105
PubChem Substance ID:
NACRES:
NA.21
Recommended Products
Quality Level
Assay
≥90.0% (CHN)
form
powder
quality
for spectrophotometric det. of Si
technique(s)
UV/Vis spectroscopy: suitable
mp
290 °C (dec.) (lit.)
SMILES string
Nc1c(O)cc(c2ccccc12)S(O)(=O)=O
InChI
1S/C10H9NO4S/c11-10-7-4-2-1-3-6(7)9(5-8(10)12)16(13,14)15/h1-5,12H,11H2,(H,13,14,15)
InChI key
RXCMFQDTWCCLBL-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
4-Amino-3-hydroxy-1-naphthalenesulfonic acid is an aromatic amino-sulfonic acid.
Application
4-Amino-3-hydroxy-1-naphthalenesulfonic acid has been used to study separation of aromatic sulfonated compounds using Ion-interaction high-performance liquid chromatography and micellar electrokinetic capillary chromatography techniques. It has also been used as a starting material which when reacted with palmitoyl chloride yields the following derivatives:
- 2′-Pentadecylnaphth[3,4-d]oxazole-1-sulfonic acid
- 4-Amino-3-(palmitoyloxy)-1-naphthalenesufonic acid
- 4-(Palmitoylamino)-3-hydroxy-1-naphthalenesulfonic acid
- 4-(Palmitoylamino)-3-(palmitoyloxy)-1-naphthalenesulfonic acid
Other Notes
Reagent for the spectrophotometric det. of silicon
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis of naphthalenesulfonic acid small molecules as selective inhibitors of the DNA polymerase and ribonuclease H activities of HIV-1 reverse transcriptase.
Mohan P
Journal of Medicinal Chemistry, 37(16), 2513-2519 (1994)
F.A. Sorrentino et al.
Microchemical Journal, Devoted to the Application of Microtechniques in All Branches of Science, 15, 441-441 (1970)
Ion-interaction high-performance liquid chromatography and micellar electrokinetic capillary chromatography: two complementary techniques for the separation of aromatic sulfonated compounds.
Angelino, S., et al.
Journal of Chromatography A, 845.1, 257-271 (1999)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service