8.56045
Fmoc-Gly-NovaSyn® TGT
for peptide synthesis, Novabiochem®
Synonym(s):
Fmoc-Glycine Resin
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
UNSPSC Code:
12352209
NACRES:
NA.22
Recommended Products
product name
Fmoc-Gly-NovaSyn® TGT, Novabiochem®
Quality Level
product line
NovaSyn® TG
Novabiochem®
form
beads
reaction suitability
reaction type: Fmoc solid-phase peptide synthesis
manufacturer/tradename
Novabiochem®
application(s)
peptide synthesis
functional group
Fmoc
storage temp.
15-25°C
Related Categories
General description
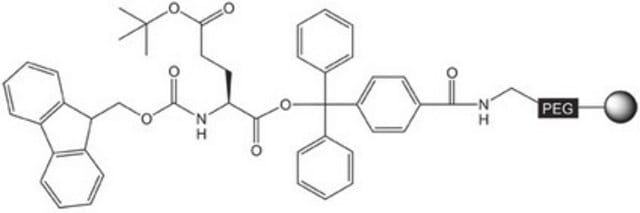
Pre-loaded resin for synthesis of peptide acids and protected peptide fragments containing a C-terminal glycine amino-acid residue by Fmoc SPPS.The base NovaSyn® TG is a composite of low cross-linked polystyrene and 3000-4000 M.W. polyethylene glycol. Peptide synthesis is carried out at the ends of the PEG chains that have been functionalized with the hyper-acid labile 4-carboxytrityl alcohol linker. This use of the bulky trityl-type linker helps prevent diketopiperazine formation that can occur during piperidine treatment of Fmoc-protected dipeptidyl resins.,,Treatment of the peptidyl resin with 20% TFE in DCM or 1% TFA in DCM cleaves the product from the resin without affecting the standard TFA-labile side-chain protecting groups. Standard TFA cleavage releases the fully deprotected peptide.
Associated Protocols and Technical Articles
Cleavage and Deprotection Protocols for Fmoc SPPS
Literature references:
[1] K. Barlos, et al. (1989) Tetrahedron Lett., 30, 3947.
[2] K. Barlos, et al. (1993) Ann. Chem., 215.
[3] R. Steinauer, et al. in “Innovations & Perspectives in Solid Phase Synthesis”, R. Epton (Ed.), Mayflower Scientific Ltd., Birmingham, 1994, pp. 689.
[4] K. Barlos & D. Gatos in “Fmoc solid phase peptide synthesis: a practical approach”, W. C. Chan & P. D.White (Eds.), Oxford University Press, Oxford, 2000, pp. 218.
Associated Protocols and Technical Articles
Cleavage and Deprotection Protocols for Fmoc SPPS
Literature references:
[1] K. Barlos, et al. (1989) Tetrahedron Lett., 30, 3947.
[2] K. Barlos, et al. (1993) Ann. Chem., 215.
[3] R. Steinauer, et al. in “Innovations & Perspectives in Solid Phase Synthesis”, R. Epton (Ed.), Mayflower Scientific Ltd., Birmingham, 1994, pp. 689.
[4] K. Barlos & D. Gatos in “Fmoc solid phase peptide synthesis: a practical approach”, W. C. Chan & P. D.White (Eds.), Oxford University Press, Oxford, 2000, pp. 218.
Application
Fmoc-Gly-NovaSyn® TGT has been used to preparehydrazinopeptides as intermediates in the semi-synthesis of glycopeptides.
Linkage
Replaces: 04-12-2711
Analysis Note
Color (visual): white to yellow to beige
Appearance of substance (visual): beads
Loading (Photometric determination of the Fmoc-chromophore liberated upon treatment with DBU/DMF): 0.10 - 0.30 mmol/g
Swelling Volume (in DMF): lot specific result
Identity (of the substitution): passes test
The base resin is PEG-PS-copoymer (90µm), functionalised with 4-carboxy-tritylchloride.
Appearance of substance (visual): beads
Loading (Photometric determination of the Fmoc-chromophore liberated upon treatment with DBU/DMF): 0.10 - 0.30 mmol/g
Swelling Volume (in DMF): lot specific result
Identity (of the substitution): passes test
The base resin is PEG-PS-copoymer (90µm), functionalised with 4-carboxy-tritylchloride.
Legal Information
NOVASYN is a registered trademark of Merck KGaA, Darmstadt, Germany
Novabiochem is a registered trademark of Merck KGaA, Darmstadt, Germany
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Innovations & Perspectives in Solid Phase Synthesis
R. Steinauer, et al.
Solid Phase Synthesis, 2nd Int. Symposium, R. Epton, ed., 689-689 (1994)
K. Barlos, et al.
Ann. Chem., 215-215 (1993)
Investigation of acyl transfer auxiliary-assisted glycoconjugation for glycoprotein semi-synthesis
Nyandoro K, et al.
Organic & Biomolecular Chemistry, 20, 8506-8514 (2022)
Veresterung von partiell geschutzten peptid-fragmenten mit harzen. Einsatz von 2-chlortritylchlorid zur synthese von Leu15-gastrin I
K. Barlos, et al.
Tetrahedron Letters, 30, 3947-3947 (1989)
Fmoc solid phase peptide synthesis: a practical approach
K. Barlos & D. Gatos
Solid Phase Peptide Synthesis: A Practical Approach, 218-218 (2000)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service