860657P
Avanti
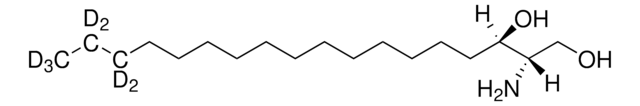
sphingosine-d7
Avanti Research™ - A Croda Brand 860657P, powder
Synonym(s):
D-erythro-sphingosine-d7
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C18H30D7NO2
CAS Number:
Molecular Weight:
306.54
UNSPSC Code:
12352211
NACRES:
NA.25
Recommended Products
form
powder
packaging
pkg of 1 × 1 mg (860657P-1mg)
pkg of 1 × 5 mg (860657P-5mg)
manufacturer/tradename
Avanti Research™ - A Croda Brand 860657P
shipped in
dry ice
storage temp.
−20°C
SMILES string
OC[C@@](N)([H])[C@@](O)([H])/C=C/CCCCCCCCCCC(C(C([2H])([2H])[2H])([2H])[2H])([2H])[2H]
General description
Sphingosine-d7 is a deuterated derivative of sphingosine. Sphingosine is an important bioactive sphingolipid metabolite. It is a 18-carbon amino alcohol derived from sphingomyelin. Sphingosine contains an unsaturated hydrocarbon chain.
Application
Sphingosine-d7 has been used as an internal standard in liquid chromatography–tandem mass spectrometry for quantitative analysis of sphingolipids in biological samples.
Biochem/physiol Actions
Sphingosine negatively regulates cell proliferation and induces apoptosis. It has an ability to regulate the activities of phospholipases, protein kinases, ion channels, cannabinoid receptor type 1 (CB-1) receptors and steroidogenic factor 1 (SF-1) receptors. Sphingosine acts as a precursor for ceramide synthesis.
Packaging
5 mL Amber Glass Screw Cap Vial (860657P-1mg)
5 mL Amber Glass Screw Cap Vial (860657P-5mg)
Legal Information
Avanti Research is a trademark of Avanti Polar Lipids, LLC
also commonly purchased with this product
Product No.
Description
Pricing
Storage Class Code
11 - Combustible Solids
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
the
Lipid Mediators and Their Metabolism in the Brain (2011)
Iulia Zoicas et al.
Cells, 9(5) (2020-05-24)
Human and murine studies identified the lysosomal enzyme acid sphingomyelinase (ASM) as a target for antidepressant therapy and revealed its role in the pathophysiology of major depression. In this study, we generated a mouse model with overexpression of Asm (Asm-tgfb)
Stephanie Schwalm et al.
The American journal of pathology, 187(11), 2413-2429 (2017-08-16)
Kidney fibrosis is a hallmark of chronic kidney disease and leads to extracellular matrix accumulation, organ scarring, and loss of kidney function. In this study, we investigated the role of sphingosine kinase-2 (SPHK2) on the progression of tubular fibrosis by
Henning Carstens et al.
Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology, 53(6), 1015-1028 (2019-12-20)
Pulmonary infections with Pseudomonas aeruginosa (P. aeruginosa) or Staphylococcus aureus (S. aureus) are of utmost clinical relevance in patients with cystic fibrosis, chronic obstructive pulmonary disease, after trauma and burn, upon ventilation or in immuno-compromised patients. Many P. aeruginosa and
Irina Alecu et al.
Journal of lipid research, 58(1), 60-71 (2016-11-23)
The 1-deoxysphingolipids (1-deoxySLs) are atypical sphingolipids (SLs) that are formed when serine palmitoyltransferase condenses palmitoyl-CoA with alanine instead of serine during SL synthesis. The 1-deoxySLs are toxic to neurons and pancreatic β-cells. Pathologically elevated 1-deoxySLs cause the inherited neuropathy, hereditary
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service