W508306
Tricarballylic acid
≥99%
Synonym(s):
1,2,3-Propanetricarboxylic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
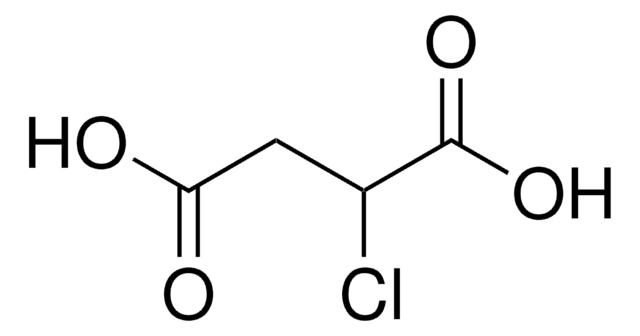
(HO2CCH2)2CHCO2H
CAS Number:
Molecular Weight:
176.12
Beilstein:
1783567
EC Number:
MDL number:
UNSPSC Code:
12164502
PubChem Substance ID:
Recommended Products
Assay
≥99%
mp
156-161 °C (lit.)
SMILES string
OC(=O)CC(CC(O)=O)C(O)=O
InChI
1S/C6H8O6/c7-4(8)1-3(6(11)12)2-5(9)10/h3H,1-2H2,(H,7,8)(H,9,10)(H,11,12)
InChI key
KQTIIICEAUMSDG-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Jeffrey M Boyd et al.
Journal of bacteriology, 194(3), 576-583 (2011-11-22)
Mutants of Salmonella enterica lacking apbC have nutritional and biochemical properties indicative of defects in iron-sulfur ([Fe-S]) cluster metabolism. An apbC mutant is unable to grow on tricarballylate as a carbon source. Based on the ability of ApbC to transfer
P C Hallson et al.
Urologia internationalis, 57(1), 43-47 (1996-01-01)
The ability of three compounds, all similar in chemical structure to citric acid, to decrease calcium crystalluria has been measured. The measurements were made in normal human urine at 37 degrees C and compared with the crystal-decreasing power of citric
Linda Bíró et al.
Journal of inorganic biochemistry, 116, 116-125 (2012-09-29)
The interaction between [Ru(η(6)-p-cym)(H(2)O)(3)](2+) and an important low molecular weight serum component, citric acid (citrH(3)), was studied with the aid of combined pH-potentiometric, (1)H NMR, (13)C NMR and electrospray ionization mass spectrometry (ESI-MS) methods in aqueous solution. For comparative purposes
M Hartl et al.
The Journal of organic chemistry, 66(11), 3678-3681 (2001-05-26)
The circular dichroism (CD) exciton chirality method was employed for the stereochemical assignment of the tricarballylic acid (TCA) side chains of fumonisin B(1) 1a (FB(1)). Using 2-naphthoate for chromophoric derivatization of the reduced TCA moieties, the absolute configuration was shown
Kathia Zaleta-Rivera et al.
Biochemistry, 45(8), 2561-2569 (2006-02-24)
Fumonisins are a group of polyketide-derived mycotoxins produced by Fusarium verticillioides, a filamentous fungus infecting corn and contaminating food and feeds. Fumonisins contain two tricarballylic esters that are critical for toxicity. Here, we present genetic and biochemical data for the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service