All Photos(1)
About This Item
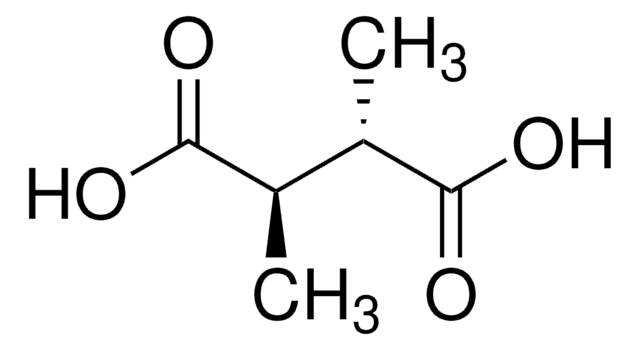
Linear Formula:
HOOCCH(CH3)CH(CH3)COOH
CAS Number:
Molecular Weight:
146.14
Beilstein:
1723932
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥99%
form
powder
SMILES string
CC(C(C)C(O)=O)C(O)=O
InChI
1S/C6H10O4/c1-3(5(7)8)4(2)6(9)10/h3-4H,1-2H3,(H,7,8)(H,9,10)
InChI key
KLZYRCVPDWTZLH-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Jannick Jacobsen et al.
Dalton transactions (Cambridge, England : 2003), 48(23), 8433-8441 (2019-05-23)
Six different chiral and achiral alkane dicarboxylic C4-acids, i.e. succinic acid (H2SUC), dl-2-methylsuccinic acid (H2MS), 2,3-dimethylsuccinic acid (H2DMS) and aspartic acid (d-, l- and dl-H2ASP), were used to obtain Ce(iv)-MOFs via microwave assisted reactions. In water-based syntheses, MOFs with three
P H Andersen et al.
Food additives and contaminants, 1(3), 283-288 (1984-07-01)
Three flavourings: dimethyl succinate, ethyl pyruvate and aconitic acid, commonly used in candy, beverages, and baked goods, were tested in the Salmonella/mammalian-microsome test. Tester strains were TA 1535, TA 100, TA 1537 and TA 98 and doses were 32, 160
G Branlant
European journal of biochemistry, 121(2), 407-411 (1982-01-01)
Aldehyde reductase I from pig liver is strongly inhibited by cyclized NADP--2-oxodiacid adducts. This result, in conjunction with those showing a strong inhibitory effect of certain diacid derivatives, such as (+/-)-dimethylsuccinic acid, towards aldehyde reductase I, suggests the presence of
P S Thomas et al.
Postgraduate medical journal, 67(783), 63-65 (1991-01-01)
Chronic lead poisoning has traditionally been treated by parenteral agents. We present a case where a comparison of ethylene diaminetetra-acetic acid was made with 2,3-dimethyl succinic acid (DMSA) which has the advantage of oral administration associated with little toxicity and
E Asante-Appiah et al.
Biochemistry, 36(29), 8710-8715 (1997-07-22)
gem-Dimethylsuccinic acid and its higher homolog, 2-methyl-2-ethylsuccinic acid (MESA) are highly potent inhibitors of both carboxypeptidase A (CPA) and B. The inhibition constant of MESA for CPA (0.11 microM for the racemic mixture) is remarkable considering the relatively simple structure
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service