All Photos(1)
About This Item
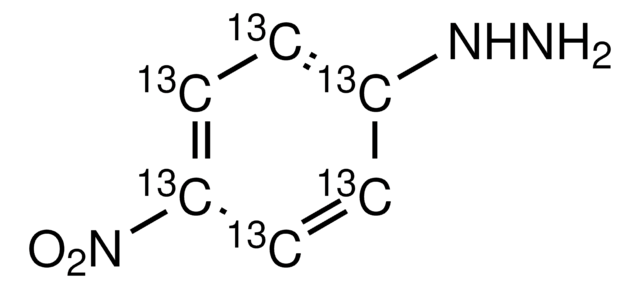
Linear Formula:
O2NC6H4NHNH2
CAS Number:
Molecular Weight:
153.14
Beilstein:
608107
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
Recommended Products
Assay
96%
form
solid
contains
≥30% water as stabilizer
mp
156 °C (dec.) (lit.)
SMILES string
NNc1ccc(cc1)[N+]([O-])=O
InChI
1S/C6H7N3O2/c7-8-5-1-3-6(4-2-5)9(10)11/h1-4,8H,7H2
InChI key
KMVPXBDOWDXXEN-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Oral - Expl. 1.1 - Eye Irrit. 2 - Flam. Sol. 2 - Skin Irrit. 2 - Skin Sens. 1 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
1 - Explosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
R V Raju et al.
Journal of AOAC International, 77(3), 748-751 (1994-05-01)
Three spectrophotometric methods were developed for the microdetermination of decamethrin in insecticidal formulations and in water. The methods are based on the hydrolysis of decamethrin with methanolic KOH to 3-phenoxybenzaldehyde; condensation of the hydrolysis product with 2,4-dinitrophenylhydrazine (2,4-DNPH), 4-nitrophenyl-hydrazine (4-NPH)
Minae Mure et al.
Journal of the American Chemical Society, 125(20), 6113-6125 (2003-06-06)
4-n-Butylamino-5-ethyl-1,2-benzoquinone (1(ox)) has been synthesized as a model compound for the LTQ (lysine tyrosyl quinone) cofactor of lysyl oxidase (LOX). At pH 7, 1(ox) has a lambda(max) at 504 nm and exists as a neutral o-quinone in contrast to a
S R Carter et al.
Journal of inorganic biochemistry, 56(2), 127-141 (1994-11-01)
An improved purification scheme for an amine oxidase from equine plasma (EPAO), a nonruminant source, is described and the protein's active-site is characterized. EPAO is dimeric and contains one Type-2 Cu(II) ion per monomer. The EPAO Cu(II) site is spectroscopically
T Huque et al.
Comparative biochemistry and physiology. B, Comparative biochemistry, 86(1), 135-139 (1987-01-01)
The total plasmalogen content of lingual and other tissues was analyzed using the iodine-addition (Method 1), the p-nitrophenylhydrazone (Method 2), and the two-dimensional thin layer chromatography procedure (Method 3). Methods 1 and 2 were simple, rapid and reproducible, yielding values
Atsuko Satoh et al.
Biochimica et biophysica acta, 1647(1-2), 272-277 (2003-04-11)
Quinohemoprotein amine dehydrogenase (QH-AmDH) catalyzes the oxidative deamination of aliphatic and aromatic amines. The enzyme from Pseudomonas putida has an alpha beta gamma heterotrimeric structure with two heme c groups in the largest alpha subunit, and a novel quinone cofactor
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service