All Photos(1)
About This Item
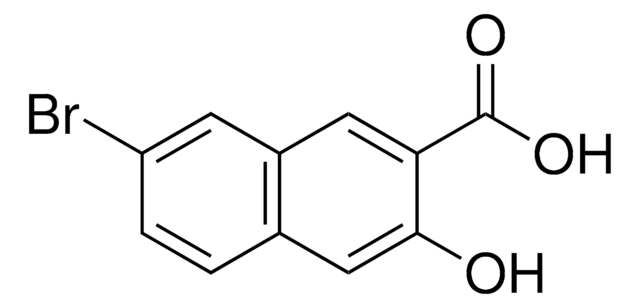
Linear Formula:
CH3OC10H6CO2H
CAS Number:
Molecular Weight:
202.21
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
mp
125-129 °C (lit.)
SMILES string
COc1c(ccc2ccccc12)C(O)=O
InChI
1S/C12H10O3/c1-15-11-9-5-3-2-4-8(9)6-7-10(11)12(13)14/h2-7H,1H3,(H,13,14)
InChI key
PMJACRPIWSINFF-UHFFFAOYSA-N
General description
1-Methoxy-2-naphthoic acid can be prepared from 1-methoxynaphthalene. 1-Methoxy-2-naphthoic acid can also be synthesized by reacting potassium tert-butoxide with 1-methoxynaphthalene and butyllithium in the presence of cyclohexane and tetrahydrofuran. It undergoes reduction in the presence of lithium to afford tetrahydronaphthoic acid.
Application
1-Methoxy-2-naphthoic acid may be used in the synthesis of 2-(1-methoxy-2-naphthyl)-4,4-dimethyl-2-oxazoline. It may also be used in the synthesis of the following compounds:
- 1-sec-butyl-2-naphthoic acid
- 1-tert-butyl-2-naphthoic acid
- 1-ethyl-2-naphthoic acid
- 1-vinyl-2-naphthoic acid
- 1-phenyl-2-naphthoic acid
- 1-(2,5-dimethylphenyl)-2-naphthoic acid
- 2′-methoxy-[1,1′-binaphthalene]-2-carboxylic acid
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
The metalation of 1-methoxynaphthalene with n-butyllithium.
Graybill BM and Shirley DA.
The Journal of Organic Chemistry, 31(4), 1221-1225 (1966)
Eva Castagnetti et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 8(4), 799-804 (2002-02-22)
Judged by its capacity to promote a hydrogen/metal permutation at an ortho position, the trifluoromethoxy group is superior to both the methoxy and trifluoromethyl groups. Moreover, like CF(3) and unlike OCH(3), OCF(3) exerts a long-range effect that still considerably lowers
Chemistry of aryloxazolines. Applications to the synthesis of lignan lactone derivatives.
Meyers AI and Avila WB.
The Journal of Organic Chemistry, 46(19), 3881-3886 (1981)
Birch Reduction of 2-Naphthoic and of ortho-Methoxynaphthoic Acids.
Eliel EL and Hoover TE.
The Journal of Organic Chemistry, 24(7), 938-942 (1959)
Regadia Aissaoui et al.
The Journal of organic chemistry, 77(1), 718-724 (2011-11-24)
Substitution of an ortho-fluoro or methoxy group in 1- and 2-naphthoic acids furnishing substituted naphthoic acids occurs in good to excellent yields upon reaction with alkyl/vinyl/aryl organolithium and Grignard reagents, in the absence of a metal catalyst without the need
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service![Dichloro[(S,S)-ethylenebis(4,5,6,7-tetrahydro-1-indenyl)]zirconium(IV)](/deepweb/assets/sigmaaldrich/product/structures/124/554/8e617376-6d1e-4ab5-af24-cd94f4ba26d3/640/8e617376-6d1e-4ab5-af24-cd94f4ba26d3.png)



![Tris[N,N-bis(trimethylsilyl)amide]yttrium](/deepweb/assets/sigmaaldrich/product/structures/867/983/5b7cb7cd-8879-49e4-a9d7-29c52aaa82a0/640/5b7cb7cd-8879-49e4-a9d7-29c52aaa82a0.png)