412015
3-Bromo-4-methoxybenzaldehyde
98%
Synonym(s):
3-Bromo-p-anisaldehyde
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
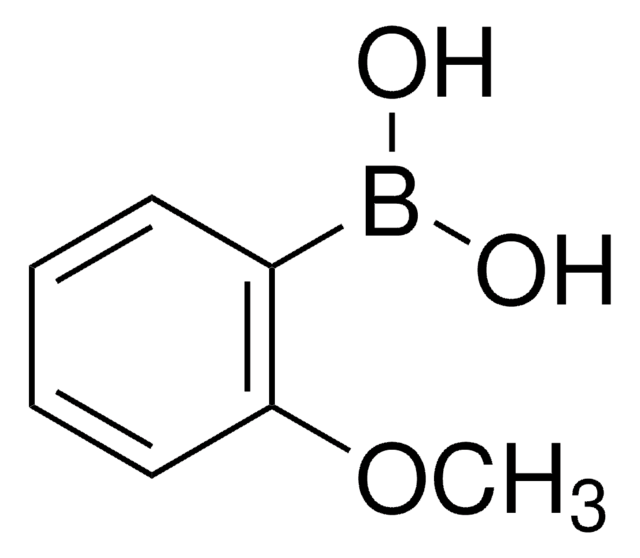
BrC6H3(OCH3)CHO
CAS Number:
Molecular Weight:
215.04
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
mp
51-54 °C (lit.)
SMILES string
COc1ccc(C=O)cc1Br
InChI
1S/C8H7BrO2/c1-11-8-3-2-6(5-10)4-7(8)9/h2-5H,1H3
InChI key
QMPNFQLVIGPNEI-UHFFFAOYSA-N
General description
3-Bromo-4-methoxybenzaldehyde is formed by the solvent-free bromination of 4-methoxybenzaldehyde using 1,3-di-n-butylimidazolium tribromide, as a brominating reagent.
Application
3-Bromo-4-methoxybenzaldehyde may be used in the following studies:
- Asymmetric synthesis of a novel β-hydroxy-α-amino acid derivative, via Mukaiyama aldol reaction.
- Synthesis of 2-(3-bromo-4-methoxyphenyl)-5-fluorobenzothiazole.
- Preparation of 5-[(Z)-2-(3-bromo-4-methoxyphenyl)vinyl]-1,2-3-trimethoxybenzene.
- Total synthesis of engelhardione.
- Starting reagent for the synthesis of (2E)-3-(3-bromo-4-methoxyphenyl)-1-(4-methylphenyl)prop-2-en-1-one.
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
22287813
10th Internatl. Conf. on Organic Synthesis, Bangalore, India, December 1994 null
Li Shen et al.
Tetrahedron letters, 52(35), 4570-4574 (2011-09-20)
The total synthesis of the macrocyclic natural product engelhardione is reported. This effort led to the structural revision of the published structure of engelhardione to that of pterocarine. The revision reflects the change of the substitution pattern of one phenyl
Yali Kong et al.
Chemistry & biology, 12(9), 1007-1014 (2005-09-27)
Targeting the microtubule system represents an attractive strategy for the development of anticancer agents. In this study, we report a class of combretastatin A-4 (CA-4) analogs derivatized with a boronic acid moiety replacing the hydroxyl group on the C-ring of
Aromatic bromination of aldehydes and ketones using 1, 3-di-n-butylimidazolium tribromide [BBIm] Br3 ionic liquids under solvent-free conditions.
Borikar SP and Daniel T.
Journal of the Iranian Chemical Society, 8(2), 531-536 (2011)
(2E)-3-(3-Bromo-4-methoxyphenyl)-1-(4-methylphenyl)prop-2-en-1-one.
Dutkiewicz g, et al.
Acta Crystallographica Section E, Structure Reports Online, 67(4), 1024-1024 (2011)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service