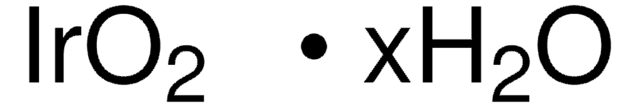208833
Ruthenium(IV) oxide hydrate
powder
Synonym(s):
Hydrous ruthenium oxide, Ruthenium dioxide hydrate
Sign Into View Organizational & Contract Pricing
All Photos(4)
About This Item
Linear Formula:
RuO2 · xH2O
CAS Number:
Molecular Weight:
133.07 (anhydrous basis)
EC Number:
MDL number:
UNSPSC Code:
12352303
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
form
powder
reaction suitability
reagent type: catalyst
core: ruthenium
SMILES string
O.O=[Ru]=O
InChI
1S/H2O.2O.Ru/h1H2;;;
InChI key
FGEKTVAHFDQHBU-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Naiqiang Yan et al.
Environmental science & technology, 45(13), 5725-5730 (2011-06-15)
Catalytic conversion of elemental mercury (Hg(0)) to its oxidized form has been considered as an effective way to enhance mercury removal from coal-fired power plants. In order to make good use of the existing selective catalytic reduction of NO(x) (SCR)
M Gonçalves et al.
Bioresource technology, 99(17), 8207-8211 (2008-05-02)
Electrochemical treatment of oleate using RuO2 and IrO2 type dimensionally stable anodes in alkaline medium was performed to develop a feasible anaerobic pre-treatment of fatty effluents. The results showed that the pre-treated solutions over RuO2 were faster degraded by anaerobic
Aleksandar R Zeradjanin et al.
ChemSusChem, 5(10), 1897-1904 (2012-08-16)
The reaction path of the Cl(2) evolution reaction (CER) was investigated by combining electrochemical and spectroscopic methods. It is shown that oxidation and reconstruction of the catalyst surface during CER is a consequence of the interaction between RuO(2) and water.
Hui Wang et al.
Journal of hazardous materials, 154(1-3), 44-50 (2007-11-13)
By using a self-made carbon/polytetrafluoroethylene (C/PTFE) O2-fed as the cathode and Ti/IrO2/RuO2 as the anode, the degradation of three organic compounds (phenol, 4-chlorophenol, and 2,4-dichlorophenol) was investigated in the diaphragm (with terylene as diaphragm material) electrolysis device by electrochemical oxidation
Bong Gill Choi et al.
ChemSusChem, 5(4), 709-715 (2012-03-24)
A facilitated electrochemical reaction at the surface of electrodes is crucial for highly efficient energy conversion and storage. Herein, various nanoparticles (NPs) including Au, Pt, Pd, Ru, and RuO(2), were synthesized in situ and directly deposited on the ionic liquid
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service