18886
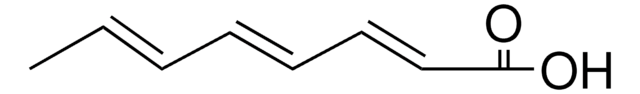
2,4-Pentadienoic acid
≥97.0% (T)
Synonym(s):
1,3-Butadiene-1-carboxylic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH2=CHCH=CHCOOH
CAS Number:
Molecular Weight:
98.10
Beilstein:
1739248
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥97.0% (T)
form
solid
contains
hydroquinone as stabilizer
mp
69-72 °C
solubility
1 M NaOH: soluble 0.5 g/10 mL, clear, brown
functional group
carboxylic acid
storage temp.
−20°C
SMILES string
OC(=O)\C=C\C=C
InChI
1S/C5H6O2/c1-2-3-4-5(6)7/h2-4H,1H2,(H,6,7)/b4-3+
InChI key
SDVVLIIVFBKBMG-ONEGZZNKSA-N
Application
2,4-Pentadienoic acid was used in preparation of chiral propargylester. It was also used in the preparation of trans 1-N-acylamino-1,3-dienes via modified Curtius procedure.
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Daichi Oguro et al.
Bioscience, biotechnology, and biochemistry, 75(8), 1502-1505 (2011-08-09)
The synthesis and sensory evaluation of enantiomeric sets of sedanenolide (1) and 3-butylphthalide (3) are described. The asymmetric synthesis was achieved via the intramolecular Diels-Alder reaction of chiral propargylester (5) which was prepared from optically active propargyl alcohol (4) and
Xiaohu Yi et al.
ChemSusChem, 10(7), 1494-1500 (2017-01-18)
A series of choline (Ch)-exchanged heteropoly acids (HOCH
trans-1-N-Acylamino-1, 3-dienes: preparation from dienoic acids.
Overman L, et al.
The Journal of Organic Chemistry, 43(11), 2164-2167 (1978)
Brian D Hudson et al.
FASEB journal : official publication of the Federation of American Societies for Experimental Biology, 26(12), 4951-4965 (2012-08-25)
When it is difficult to develop selective ligands within a family of related G-protein-coupled receptors (GPCRs), chemically engineered receptors activated solely by synthetic ligands (RASSLs) are useful alternatives for probing receptor function. In the present work, we explored whether a
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service