All Photos(3)
About This Item
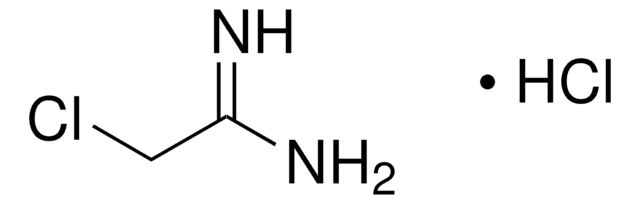
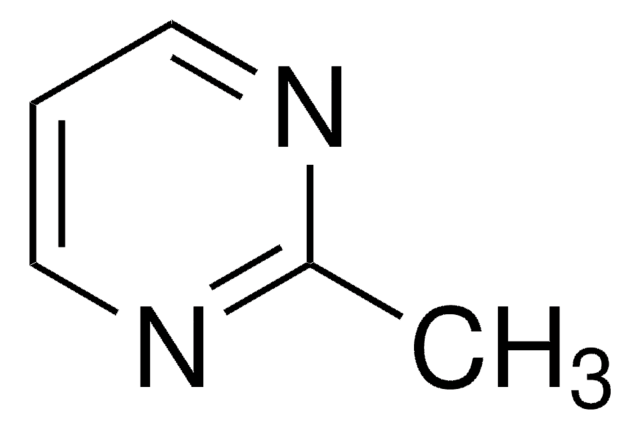
Linear Formula:
CH3C(=NH)NH2 · HCl
CAS Number:
Molecular Weight:
94.54
Beilstein:
3591762
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
95%
form
solid
mp
165-170 °C (lit.)
SMILES string
Cl[H].CC(N)=N
InChI
1S/C2H6N2.ClH/c1-2(3)4;/h1H3,(H3,3,4);1H
InChI key
WCQOBLXWLRDEQA-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Acetamidine hydrochloride is an amidine salt and its conversion to 2,4,6-trimethyl-sym-triazine has been studied.
Application
Acetamidine hydrochloride was used in the preparation of decarboxyectoine. It was also used in the synthesis of ethyl 4-(4-hydroxyphenyl)methylidene-2-methyl-5-oxo-1-imidazolacetate.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Michael Schnoor et al.
Biochemical and biophysical research communications, 322(3), 867-872 (2004-09-01)
Different substances such as dimethyl sulfoxide, tetramethylene sulfoxide, 2-pyrollidone, and the naturally occurring compatible solute betaine enhance PCR amplification of GC-rich DNA templates with high melting temperatures. In particular, cyclic compatible solutes outperform traditional PCR enhancers. We therefore investigated the
H Niwa et al.
Proceedings of the National Academy of Sciences of the United States of America, 93(24), 13617-13622 (1996-11-26)
The jellyfish Aequorea victoria possesses in the margin of its umbrella a green fluorescent protein (GFP, 27 kDa) that serves as the ultimate light emitter in the bioluminescence reaction of the animal. The protein is made up of 238 amino
Synthesis of the sym-Triazine System. I. Trimerization and Cotrimerization of Amidines.
Schaefer FC, et al.
Journal of the American Chemical Society, 81(6), 1466-1470 (1959)
Selective inhibition of iNOS by benzyl- and dibenzyl derivatives of N-(3-aminobenzyl)acetamidine.
Marialuigia Fantacuzzi et al.
ChemMedChem, 6(7), 1203-1206 (2011-05-14)
E Antonini et al.
Biochemistry, 21(10), 2477-2482 (1982-05-11)
Kinetic studies have been carried out with a well-characterized preparation of human urinary (h.u.) kallikrein using chromogenic substrates. Steady-state and pre-steady-state data for h.u. kallikrein catalyzed hydrolysis of N alpha-carbobenzoxy-L-lysine p-nitrophenyl ester (ZLysONp) and of N alpha-carbobenzoxy-L-alanine p-nitrophenyl ester (ZAlaONp)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service