102261
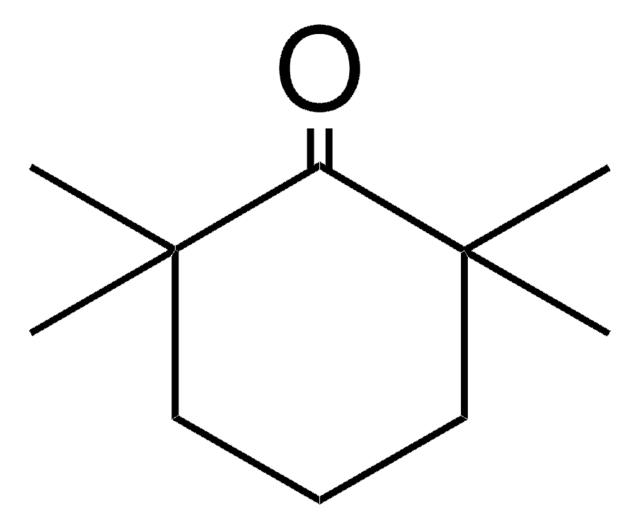
2,6-Dimethylcyclohexanone, mixture of isomers
98%
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(CH3)2C6H8(=O)
CAS Number:
Molecular Weight:
126.20
Beilstein:
1099000
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
refractive index
n20/D 1.447 (lit.)
bp
174-176 °C (lit.)
density
0.925 g/mL at 25 °C (lit.)
SMILES string
CC1CCCC(C)C1=O
InChI
1S/C8H14O/c1-6-4-3-5-7(2)8(6)9/h6-7H,3-5H2,1-2H3
InChI key
AILVYPLQKCQNJC-UHFFFAOYSA-N
Related Categories
General description
2,6-Dimethylcyclohexanone, mixture of isomers is a clear, light yellow liquid. The adsorption of traces of 2,6-dimethylcyclohexanone, a volatile organic compound, from liquid toluene was studied. cis- and trans-isomers of 2,6-Dimethylcyclohexanonereacts with hydroxylamine hydrochloride and potassium hydroxide in methanol solution gives cis- and trans-dimethylcyclohexanone oxime .
Application
2,6-Dimethylcyclohexanone was used in tetraoxane synthesis and in evaluation of their anti-malarial activity.
Signal Word
Warning
Hazard Statements
Hazard Classifications
Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
123.8 °F - closed cup
Flash Point(C)
51 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
K J McCullough et al.
Journal of medicinal chemistry, 43(6), 1246-1249 (2000-03-29)
Two tetramethyl-substituted dispiro-1,2,4,5-tetraoxanes (7,8,15, 16-tetraoxadispiro[5.2.5.2]hexadecanes) 3 and 4 were designed as metabolically stable analogues of the dimethyl-substituted dispiro-1, 2,4,5-tetraoxane prototype WR 148999 (2). For a positive control we selected the sterically unhindered tetraoxane 5 (7,8,15, 16-tetraoxadispiro[5.2.5.2]hexadecane), devoid of any substituents.
Adsorption of Volatile Organic Compounds. Experimental and Theoretical Study.
Brunchi CC, et al.
Industrial & Engineering Chemistry Research, 51(51), 16697-16708 (2012)
Photochemistry of Oximes III. The Photochemical Beckmann Rearrangement.
Cunningham M, et al.
Canadian Journal of Chemistry, 49(17), 2891-2896 (1971)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service