E5389
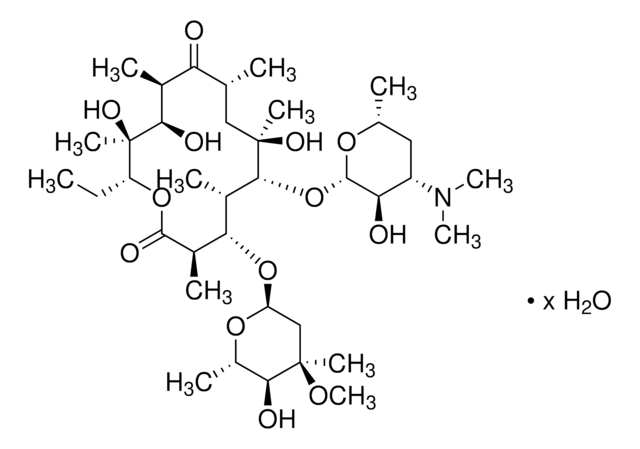
Erythromycin
powder, suitable for cell culture, BioReagent
Synonym(s):
E-Mycin, Erythrocin
About This Item
Recommended Products
product name
Erythromycin, BioReagent, suitable for cell culture
product line
BioReagent
form
powder
potency
≥850 μg per mg
technique(s)
cell culture | mammalian: suitable
impurities
≤0.1 EU/mg endotoxin
color
white
mp
133 °C
solubility
2 M HCl: 50 mg/mL (Stock solutions should be stored at 2-8 °C. Stable at 37 °C for 3 days.)
ethanol: soluble (Stock solutions should be stored at 2-8 °C. Stable at 37 °C for 3 days.)
antibiotic activity spectrum
Gram-negative bacteria
Gram-positive bacteria
Mode of action
protein synthesis | interferes
SMILES string
CC[C@H]1OC(=O)[C@H](C)[C@@H](O[C@H]2C[C@@](C)(OC)[C@@H](O)[C@H](C)O2)[C@H](C)[C@@H](O[C@@H]3O[C@H](C)C[C@@H]([C@H]3O)N(C)C)[C@](C)(O)C[C@@H](C)C(=O)[C@H](C)[C@@H](O)[C@]1(C)O
InChI
1S/C37H67NO13/c1-14-25-37(10,45)30(41)20(4)27(39)18(2)16-35(8,44)32(51-34-28(40)24(38(11)12)15-19(3)47-34)21(5)29(22(6)33(43)49-25)50-26-17-36(9,46-13)31(42)23(7)48-26/h18-26,28-32,34,40-42,44-45H,14-17H2,1-13H3/t18-,19-,20+,21+,22-,23+,24+,25-,26+,28-,29+,30-,31+,32-,34+,35-,36-,37-/m1/s1
InChI key
ULGZDMOVFRHVEP-RWJQBGPGSA-N
Gene Information
human ... ABCB1(5243) , CYP3A4(1576) , MLNR(2862)
mouse ... Abcb1a(18671) , Abcb1b(18669)
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- as a supplement for nutrient broth medium for culturing green fluorescent protein (GFP)- expressing E. coli
- as a model drug to determine small intestinal (SMI) microtissue viability using the MTT assay{254
- as an antibiotic to study the treatment strategies of chronic infections
Biochem/physiol Actions
Antimicrobial Spectrum: This product acts against both gram-negative and gram-positive bacteria.
Caution
Preparation Note
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Antibiotics targeting bacterial ribosomes disrupt protein synthesis, a key process in bacterial growth inhibition.
Antibiotics targeting bacterial ribosomes disrupt protein synthesis, a key process in bacterial growth inhibition.
Antibiotics targeting bacterial ribosomes disrupt protein synthesis, a key process in bacterial growth inhibition.
Antibiotics targeting bacterial ribosomes disrupt protein synthesis, a key process in bacterial growth inhibition.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service