PHR1039
Erythromycin
Pharmaceutical Secondary Standard; Certified Reference Material
About This Item
Recommended Products
grade
certified reference material
pharmaceutical secondary standard
Quality Level
Agency
traceable to BP 794
traceable to Ph. Eur. E1305000
traceable to USP 1242000
API family
erythromycin
CofA
current certificate can be downloaded
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
application(s)
pharmaceutical (small molecule)
format
neat
storage temp.
-10 to -25°C
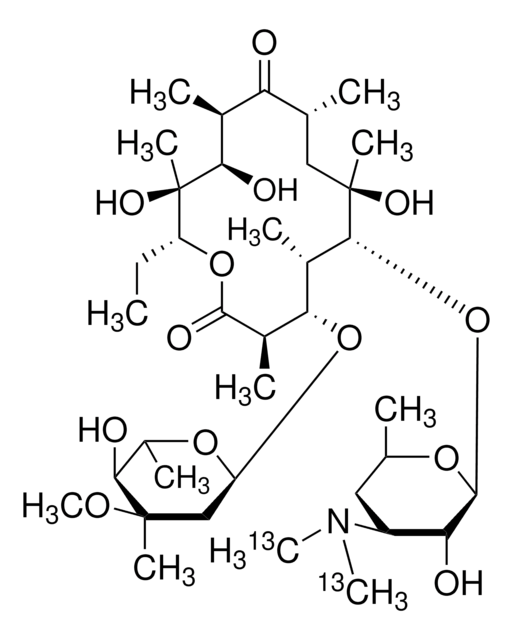
SMILES string
CC[C@H]1OC(=O)[C@H](C)[C@@H](O[C@H]2C[C@@](C)(OC)[C@@H](O)[C@H](C)O2)[C@H](C)[C@@H](O[C@@H]3O[C@H](C)C[C@@H]([C@H]3O)N(C)C)[C@](C)(O)C[C@@H](C)C(=O)[C@H](C)[C@@H](O)[C@]1(C)O
InChI
1S/C37H67NO13/c1-14-25-37(10,45)30(41)20(4)27(39)18(2)16-35(8,44)32(51-34-28(40)24(38(11)12)15-19(3)47-34)21(5)29(22(6)33(43)49-25)50-26-17-36(9,46-13)31(42)23(7)48-26/h18-26,28-32,34,40-42,44-45H,14-17H2,1-13H3/t18-,19-,20+,21+,22-,23+,24+,25-,26+,28-,29+,30-,31+,32-,34+,35-,36-,37-/m1/s1
InChI key
ULGZDMOVFRHVEP-RWJQBGPGSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Erythromycin is a complex macrolide antibiotic drug that exhibits a bacteriostatic activity. It is generally employed in human and veterinary medicines owing to its potential activity against gram positive and a few gram negative strains.
Application
Biochem/physiol Actions
Antimicrobial Spectrum: This product acts against both gram-negative and gram-positive bacteria.
Caution
Preparation Note
Footnote
Legal Information
These secondary standards offer multi-traceability to the USP, EP (PhEur) and BP primary standards, where they are available. This product was designed, produced and verified for accuracy and stability in accordance with ISO/IEC 17025:2005 (AClass Cert AT-1467), ISO GUIDES 34:2009 (AClass Cert AR-1470).
related product
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Comparative analysis of Supel™ BioSPME 96-Pin device with a rapid equilibrium dialysis technique for accuracy of measured values, sample cleanliness, and workflow time in drug protein binding.
Comparative analysis of Supel™ BioSPME 96-Pin device with a rapid equilibrium dialysis technique for accuracy of measured values, sample cleanliness, and workflow time in drug protein binding.
Comparative analysis of Supel™ BioSPME 96-Pin device with a rapid equilibrium dialysis technique for accuracy of measured values, sample cleanliness, and workflow time in drug protein binding.
Comparative analysis of Supel™ BioSPME 96-Pin device with a rapid equilibrium dialysis technique for accuracy of measured values, sample cleanliness, and workflow time in drug protein binding.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service





![(1S,6R)-6-HYDROXY-5-METHYLENE-1-[(1S)-1,2,3-TRIHYDROXY-2-METHYLPROPYL]-2-OXA-7,9-DIAZABICYCLO[4.2.2]DECANE-8,10-DIONE AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/271/983/0a99c1ad-6200-4f41-b31f-bd929e66599d/640/0a99c1ad-6200-4f41-b31f-bd929e66599d.png)