Analysis: Activated Nucleotide Sugars by LC-MS/MS
Introduction
Nucleotide sugars, or nucleotide activated sugars, are highly energetic forms of monosaccharides that act as key metabolites in glycosylation reactions, during which the glycosyl group from the activated sugars is transferred to an acceptor molecule, e.g. a protein.
In mammals, the most common nucleotide for sugar activation is uridine diphosphate (UDP), which is found in combination with Glucose (Glc), Galactose (Gal), N-Acetylglucosamine (GlcNAc), N-Acetylgalactosamine (GalNAc), Glucuronic acid (GlcA) and Xylose (Xyl). Furthermore, Guanosine diphosphate (GDP) linked to Mannose (Man) and Fucose (Fuc) as well as Cytidine monophosphate (CMP) linked to sialic acid (Neu5Ac) are used for glycan assembly. Plants and bacteria utilize an even larger variety of nucleotides and sugars.
Understanding the nucleotide sugar metabolism is of interest in different scientific areas, e.g. for the production of glycosylated therapeutic proteins in cell culture, where a sufficient glycosylation needs to be ensured in order to obtain a product of high quality and efficacy.1 For this purpose, a sensitive, quantification method is needed that is capable of simultaneously analyzing a set of polar analytes with similar structures and physicochemical properties. In this application note, an LC method using a Supel™ Carbon LC column in combination with selective MS/MS detection is described.
Experimental Conditions
The used conditions for the analysis of 11 nucleotide activated sugars in a standard solution with a concentration of 80 µM each are described in Table 1 & 2.
Results
The 5 cm x 3 mm I.D. Supel™ Carbon LC column provided, under reversed phase conditions, good retention of the 11 activated nucleotide sugar compounds in the applied standard solution (Figure 1 & Table 3).
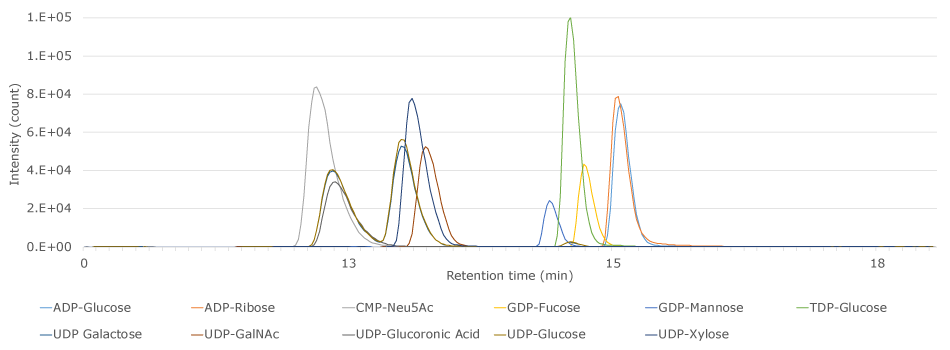
Figure 1.LC-MS/MS Analysis of nucleotide activated sugars on a Supel™ Carbon LC column.
Conclusion
This application demonstrated the use and suitability of the Supel™ Carbon LC column for the analysis of 11 nucleotide activated sugars. The group of highly polar compounds with similar structures and physicochemical properties was sufficiently retained and separated on the carbon LC column to be selectively detected with the described MS/MS method. Its ability to retain polar compounds makes the Supel™ Carbon LC column a viable choice for the analysis and quantitation of nucleotide activated sugars under reversed phase (RP) conditions.
See more information about the Supel™ Carbon LC column at SigmaAldrich.com/carbonlc
References
Para continuar lendo, faça login ou crie uma conta.
Ainda não tem uma conta?