P1781
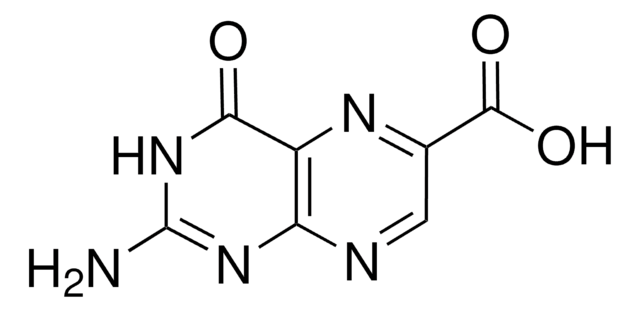
Pteroic acid
≥93%
Synonym(s):
4-{[(2-Amino-4-hydroxypteridin-6-yl)methyl]amino}benzoic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C14H12N6O3
CAS Number:
Molecular Weight:
312.28
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥93%
form
powder
SMILES string
NC1=NC(=O)C2=NC(CNc3ccc(cc3)C(O)=O)=CNC2=N1
InChI
1S/C14H12N6O3/c15-14-19-11-10(12(21)20-14)18-9(6-17-11)5-16-8-3-1-7(2-4-8)13(22)23/h1-4,6,16H,5H2,(H,22,23)(H3,15,17,19,20,21)
InChI key
JOAQINSXLLMRCV-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Pteroic acid can be used as a reactant to synthesize:
- N-trifluoroacetyl pteroic acid by reacting with trifluoroacetic anhydride via acylation.
- bis-Decyl chain derivative of pteroic acid through photocleavage reaction.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Maciej Adamczyk et al.
Bioorganic & medicinal chemistry letters, 14(9), 2313-2317 (2004-04-15)
N(10)-Trifluoroacetylpteroic acid was conjugated to chemiluminescent N-sulfonylacridinium-9-carboxamide labels at the N(10) or 9-position carboxamide. Upon binding to folate binding protein the light output of the N(10) derivative (9) was quenched up to 62% upon triggering with basic peroxide, while the
Application of halogeno-ketones to the synthesis of pteridines, including pteroic acid.
F E KING et al.
Nature, 164(4170), 574-574 (1949-10-01)
H S Tillinghast et al.
Journal of cell science, 87 ( Pt 1), 45-53 (1987-02-01)
Following a previous study indicating a sensitivity to folate during the developmental phase of Dictyostelium discoideum, a series of pteridines were investigated for their ability to induce amoebal chemotaxis during development of this organism. Several compounds were found to resemble
Kerim Babaoglu et al.
Structure (London, England : 1993), 12(9), 1705-1717 (2004-09-03)
Dihydropterate synthase (DHPS) is the target for the sulfonamide class of antibiotics, but increasing resistance has encouraged the development of new therapeutic agents against this enzyme. One approach is to identify molecules that occupy the pterin binding pocket which is
R L Stephens et al.
The Journal of pharmacology and experimental therapeutics, 239(3), 627-633 (1986-12-01)
Folic acid (FA) and 5-formyltetrahydrofolic acid (FTHF) have been shown previously to produce a marked stimulation of locomotor activity after bilateral injection into the rat nucleus accumbens. This study was designed to determine whether the hypermotility response produced by the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service