900664
Poly(D,L-lactide-co-glycolide)(50:50)-b-poly(ethylene glycol)
10k-2k
Synonym(s):
PLGA-b-PEG, PLGA-PEG
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
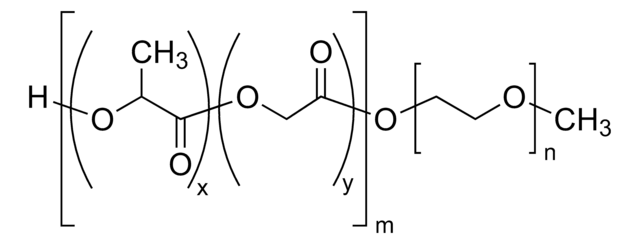
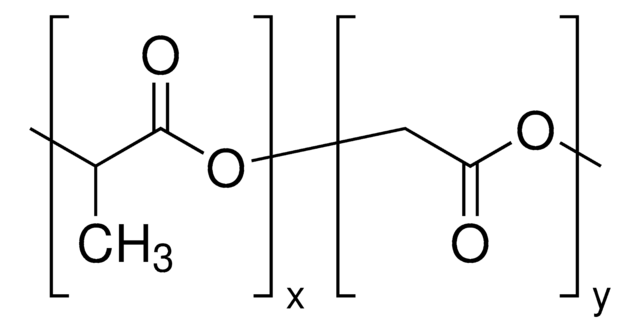
H[(C3H4O2)x(C2H2O2)y]mO[C2H4O]nCH3
UNSPSC Code:
12162002
NACRES:
NA.23
Recommended Products
Related Categories
Application
Biocompatible, amphiphilic block copolymer composed of a hydrophilic PEG block and a hydrophobic poly(D,L-lactide-co-glycolide) (PLGA) block. These materials have been used in control release and nanoparticle formulation for drug encapsulation and delivery applications. Well-defined materials with varying properties can be prepared by controlling the relative length of each polymer block. Hydroxyl termination allows for facile further chemical modification of these materials.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Yihan Xu et al.
Journal of biomedical materials research. Part B, Applied biomaterials, 105(6), 1692-1716 (2016-04-22)
Poly (lactic-co-glycolic acid) (PLGA) copolymers have been broadly used in controlled drug release applications. Because these polymers are biodegradable, they provide an attractive option for drug delivery vehicles. There are a variety of material, processing, and physiological factors that impact
R Gref et al.
Science (New York, N.Y.), 263(5153), 1600-1603 (1994-03-18)
Injectable nanoparticulate carriers have important potential applications such as site-specific drug delivery or medical imaging. Conventional carriers, however, cannot generally be used because they are eliminated by the reticulo-endothelial system within seconds or minutes after intravenous injection. To address these
Fabienne Danhier et al.
Journal of controlled release : official journal of the Controlled Release Society, 133(1), 11-17 (2008-10-28)
The purpose of this study was to develop Cremophor EL-free nanoparticles loaded with Paclitaxel (PTX), intended to be intravenously administered, able to improve the therapeutic index of the drug and devoid of the adverse effects of Cremophor EL. PTX-loaded PEGylated
Miles A Miller et al.
Nature communications, 6, 8692-8692 (2015-10-28)
Therapeutic nanoparticles (TNPs) aim to deliver drugs more safely and effectively to cancers, yet clinical results have been unpredictable owing to limited in vivo understanding. Here we use single-cell imaging of intratumoral TNP pharmacokinetics and pharmacodynamics to better comprehend their
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service