274852
N-Benzoyl-N-phenylhydroxylamine
98%
Sinónimos:
N-Hydroxy-N-phenylbenzamide, N-Phenylbenzohydroxamic acid
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
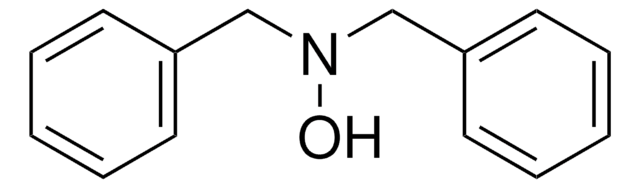
Fórmula lineal:
C6H5CON(OH)C6H5
Número de CAS:
Peso molecular:
213.23
Beilstein:
2212449
Número CE:
Número MDL:
Código UNSPSC:
12352100
ID de la sustancia en PubChem:
NACRES:
NA.22
Productos recomendados
Nivel de calidad
Ensayo
98%
Formulario
powder
mp
118-120 °C (lit.)
temp. de almacenamiento
2-8°C
cadena SMILES
ON(c1ccccc1)C(=O)c2ccccc2
InChI
1S/C13H11NO2/c15-13(11-7-3-1-4-8-11)14(16)12-9-5-2-6-10-12/h1-10,16H
Clave InChI
YLYIXDZITBMCIW-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
Descripción general
The mechanism of the reaction of N-benzoyl-N-phenylhydroxylamine with vanadium(IV) was studied.
Aplicación
N-Benzoyl-N-phenylhydroxylamine was used as a complexing agent for studying the dispersive liquid-liquid microextraction based on solidification of floating organic drop (DLLME-SFO) behavior of vanadium (V). It was used as an extractant in the determination of beryllium in natural waters.
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
Eyeshields, Gloves, type N95 (US)
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Xiaoshan Huang et al.
International journal of analytical chemistry, 2018, 8045324-8045324 (2018-08-30)
A new sensitive method for antimony (III) determination by graphite furnace atomic absorption spectrometry (GFAAS) has been developed by using N-benzoyl-N-phenylhydroxylamine (BPHA) and 1-butyl-3-methylimidazolium hexafluorophosphate ([C4mim][PF6]) single drop microextraction. The single drop microextraction (SDMM) system is more competitive compared with
Reaction mechanism of N-benzoyl-N-phenylhydroxylamine with vanadium(IV) in the weakly acidic medium.
Z Nan
Talanta, 52(5), 785-789 (2008-10-31)
The N-benzoyl-N-phenylhydroxylamine(BPHA)-V(IV) system, if kept de-aerated, gives no color reaction. In an open vessel a color reaction does take place. This reaction was studied in solution by spectrophotometry, and the product prepared as solid by scanning thermal analysis, X-ray photoelectron
Jie Li et al.
Talanta, 81(3), 954-958 (2010-03-20)
A simple and rapid analytical method for determining the concentration of rhenium in molybdenite for Re-Os dating was developed. The method used isotope dilution-inductively coupled plasma-mass spectrometry (ID-ICP-MS) after the removal of major matrix elements (e.g., Mo, Fe, and W)
L C Robles et al.
The Analyst, 116(7), 735-737 (1991-07-01)
A procedure for the determination of beryllium in natural waters is proposed. A solvent extraction step was performed in order to overcome interferences and isolate beryllium before it was atomized by direct nebulization of the organic phase in a dinitrogen
Zhefeng Fan
Analytica chimica acta, 585(2), 300-304 (2007-03-28)
A simple and sensitive method for using electrothermal atomic absorption spectrometry (ET AAS) with Rh as permanent modifier determination of Sb(III) and total Sb after separation and preconcentration by N-benzoyl-N-phenylhydroxylamine (BPHA)-chloroform single drop has been developed. Parameters, such as pyrolysis
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico