254916
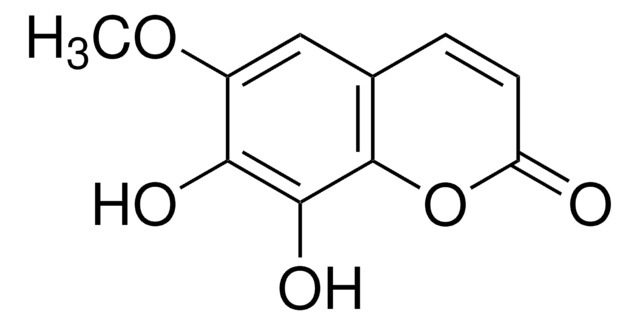
7,8-Dihydroxy-6-methoxycoumarin
98%
Sinónimos:
Fraxetin
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C10H8O5
Número de CAS:
Peso molecular:
208.17
Número CE:
Número MDL:
Código UNSPSC:
12162002
ID de la sustancia en PubChem:
NACRES:
NA.23
Productos recomendados
Nivel de calidad
Ensayo
98%
Formulario
powder
mp
230-231 °C (lit.)
cadena SMILES
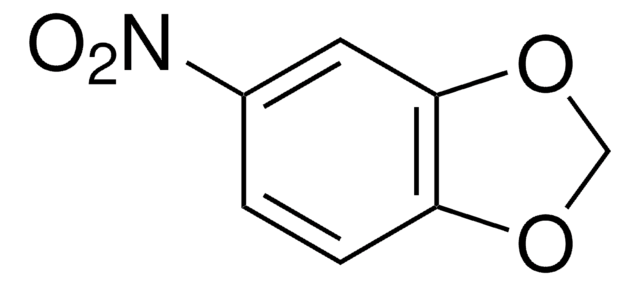
COc1cc2C=CC(=O)Oc2c(O)c1O
InChI
1S/C10H8O5/c1-14-6-4-5-2-3-7(11)15-10(5)9(13)8(6)12/h2-4,12-13H,1H3
Clave InChI
HAVWRBANWNTOJX-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Descripción general
7,8-Dihydroxy-6-methoxycoumarin is a versatile compound known for its unique fluorescence properties and its role as a photoinitiator in polymerization processes. It exhibits high reactivity under UV light, making it an ideal candidate for applications in the polymerization industry, particularly in the formulation of coatings, adhesives, and inks. It is also used in biomedical applications, such as drug delivery due to its biocompatibility.
Aplicación
7,8-Dihydroxy-6-methoxycoumarincan be used as a UV absorber in polymer formulations. Its ability to absorbultraviolet light helps in protecting polymers from degradation caused by UVradiation, thereby improving their longevity and stability.
Palabra de señalización
Warning
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Acute Tox. 4 Oral
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
dust mask type N95 (US), Eyeshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
María Isabel Sánchez-Reus et al.
Neuroscience research, 53(1), 48-56 (2005-07-06)
Fraxetin belongs to an extensive group of natural phenolic anti-oxidants. In the present study, using a human neuroblastoma SH-SY5Y cells, we have investigated the protective effects of this compound on modifications in endogenous reduced glutathione (GSH), intracellular oxygen species (ROS)
Po-Lin Kuo et al.
International immunopharmacology, 6(7), 1167-1175 (2006-05-23)
The survival of osteoblast cells is one of the determinants of the development of osteoporosis in patients with inflamed synovium, such as in rheumatoid arthritis (RA). By means of alkaline phosphatase (ALP) activity and osteocalcin ELISA assay, we have shown
Joana Terés et al.
Plant, cell & environment, 42(8), 2384-2398 (2019-04-25)
High soil carbonate limits crop performance especially in semiarid or arid climates. To understand how plants adapt to such soils, we explored natural variation in tolerance to soil carbonate in small local populations (demes) of Arabidopsis thaliana growing on soils
S Martín-Aragón et al.
Zeitschrift fur Naturforschung. C, Journal of biosciences, 52(1-2), 55-59 (1997-01-01)
We have evaluated the effects of an oral treatment of mice with fraxetin (25 mg/kg for 30 days) on the glutathione system (GSH, GSSG, and GSSG/GSH ratio as stress index), glutathione reductase (GR) and glutathione peroxidase (GPx) in liver supernatants
Phuong Thien Thuong et al.
Biological & pharmaceutical bulletin, 32(9), 1527-1532 (2009-09-02)
Atherosclerosis is main cause of arteriosclerosis. The pivotal role of low-density lipoprotein (LDL) oxidation in atherogenesis suggests antioxidants may help prevent cardiovascular disease. Fraxinus rhynchophylla DENCE (Oleaceae) is a traditional medicinal plant from East Asia. During the course of characterizing
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico