161179
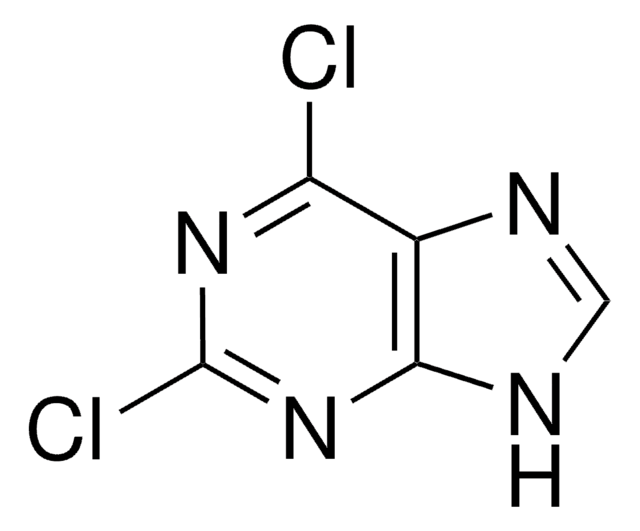
6-Chloropurine
≥99%
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C5H3ClN4
Número de CAS:
Peso molecular:
154.56
Beilstein/REAXYS Number:
5774
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
assay
≥99%
form
powder
mp
>300 °C (dec.) (lit.)
solubility
DMF: soluble 5%, clear, colorless to yellow
SMILES string
Clc1ncnc2[nH]cnc12
InChI
1S/C5H3ClN4/c6-4-3-5(9-1-7-3)10-2-8-4/h1-2H,(H,7,8,9,10)
InChI key
ZKBQDFAWXLTYKS-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
General description
The acid-catalyzed reaction of 6-chloropurine with 3,4-di-O-acetyl-D-xylal has been investigated.
Application
6-Chloropurine has been used in the preparation of 9-alkylpurines via alkylation with various substituted alkyl halides in DMSO. It was also used in the preparation of 6-succinoaminopurine.
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Synthesis of Potential Anticancer Agents. XXVI. The Alkylation of 6-Chloropurine2.
Montgomery JA and Temple Jr C.
Journal of the American Chemical Society, 83(3), 630-635 (1961)
Synthesis of 6-succinoaminopurine.
C E CARTER
The Journal of biological chemistry, 223(1), 139-146 (1956-11-01)
Heterocyclic N-glycosides-V: Synthesis of unsaturated N-glycosides from 6-chloropurine and derivatives of d-xylal and l-arabinal. A conformational NMR study.
Fuertes M, et al.
Tetrahedron, 26(20), 4823-4837 (1970)
Hai-Ming Guo et al.
Bioorganic & medicinal chemistry letters, 20(10), 3098-3102 (2010-04-20)
The synthesis and fluorescence properties of novel purine analogues linked azacrown ether at C6 position were investigated. These new purine analogues could be prepared from a series of 6-chloropurines and showed selective and efficient signaling behaviors toward micromolar concentration of
V Gurvich et al.
Nucleosides & nucleotides, 18(10), 2327-2333 (2000-01-05)
Tetrabutylammonium triphenydifluorosilicate (TBAT) has been found to be a useful reagent for the conversion of 6-chloropurine nucleosides to 6-fluoropurine derivatives. The 6-chloropurine nucleosides were reacted with trimethylamine to form quaternary trimethylammonium salts which were treated in situ with TBAT in
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico