148113
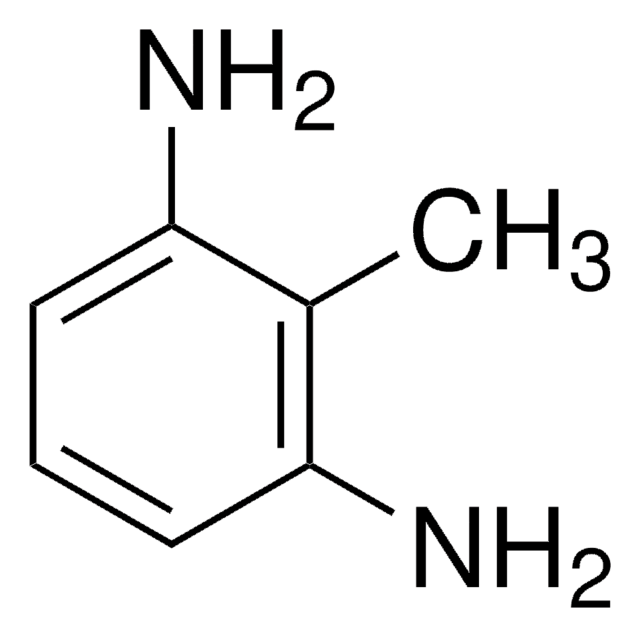
2,6-Diaminotoluene
97%
Sinónimos:
2,6-Toluenediamine, 2,6-Tolylenediamine, 2-Methyl-m-phenylenediamine
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula lineal:
CH3C6H3(NH2)2
Número de CAS:
Peso molecular:
122.17
Beilstein:
2079476
Número CE:
Número MDL:
Código UNSPSC:
12162002
ID de la sustancia en PubChem:
NACRES:
NA.23
Productos recomendados
Nivel de calidad
Ensayo
97%
Formulario
solid
mp
104-106 °C (lit.)
cadena SMILES
Cc1c(N)cccc1N
InChI
1S/C7H10N2/c1-5-6(8)3-2-4-7(5)9/h2-4H,8-9H2,1H3
Clave InChI
RLYCRLGLCUXUPO-UHFFFAOYSA-N
Palabra de señalización
Warning
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Acute Tox. 4 Dermal - Acute Tox. 4 Oral - Aquatic Chronic 2 - Muta. 2 - Skin Sens. 1
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
M L Cunningham et al.
Environmental health perspectives, 104 Suppl 3, 683-686 (1996-05-01)
The aromatic amines 2,4-diaminotoluene (2,4-DAT) and 2,6-diaminotoluene (2,6-DAT) are structural isomers that have been extensively studied for their mutagenic and carcinogenic characteristics. Both compounds are rapidly absorbed after oral administration and are equally mutagenic in the Ames test; however, 2,4-DAT
P Lind et al.
The Analyst, 122(1), 51-56 (1997-01-01)
Blood and urine samples were collected from six workers and two volunteers exposed to thermal degradation products from toluene diisocyanate (TDI)-based polyurethane (PUR) before and during the summer vacation. Air samples were collected on filters impregnated with 9-(N-methylaminomethyl)anthracene. The concentrations
Diaminotoluenes induce intrachromosomal recombination and free radicals in Saccharomyces cerevisiae.
R J Brennan et al.
Mutation research, 381(2), 251-258 (1998-01-22)
The carcinogenicity of aniline-based aromatic amines is poorly reflected by their activity in short-term mutagenicity assays such as the Salmonella typhimurium reverse mutation (Ames) assay. More information about the mechanism of action of such carcinogens is needed. Here we report
Differential in vivo mutagenicity of the carcinogen/non-carcinogen pair 2,4- and 2,6-diaminotoluene.
J J Hayward et al.
Carcinogenesis, 16(10), 2429-2433 (1995-10-01)
The aromatic amines 2,4-diaminotoluene (2,4-DAT) and 2,6-diaminotoluene (2,6-DAT) are structural isomers that have been extensively studied for their mutagenic and carcinogenic characteristics. Both compounds are equally mutagenic in the Ames/Salmonella assay in the presence of S9. However, the differences in
M Taningher et al.
Toxicology, 99(1-2), 1-10 (1995-05-05)
Among aminoaromatics, 2,4-diaminotoluene (2,4-DAT) and 2,6-diaminotoluene (2,6-DAT) represent a conflicting couple of isomers; despite showing the same structural alert to DNA reactivity (and thus potential genotoxicity), they are different in terms of carcinogenicity. Of the two, 2,4-DAT alone is a
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico