All Photos(1)
About This Item
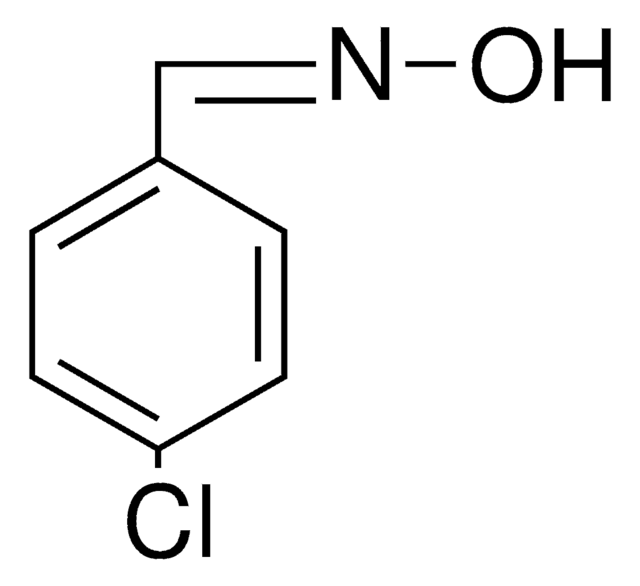
Linear Formula:
ClC6H4CH=NOH
CAS Number:
Molecular Weight:
155.58
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
mp
73-76 °C (lit.)
functional group
amine
chloro
oxime
SMILES string
O\N=C\c1ccccc1Cl
InChI
1S/C7H6ClNO/c8-7-4-2-1-3-6(7)5-9-10/h1-5,10H/b9-5+
InChI key
FZIVKDWRLLMSEJ-WEVVVXLNSA-N
Related Categories
General description
2-Chlorobenzaldehyde oxime is also known as o-chlorobenzaldehyde oxime. It can be synthesized by reacting 2-chlorobenzaldehyde and hydroxylamine hydrochloride.
Application
2-Chlorobenzaldehyde oxime may be used in the preparation of:
- 2-chlorobenzaldehyde under different reaction conditions
- methyl 3-(2-chlorophenyl)-5-[1-(4-methoxybenzyloxy)-ethyl]isoxazole-4-carboxylate
- dimethyl 3-(2-chlorophenyl)isoxazole-4,5-dicarboxylate
- [3-(2-chlorophenyl)isoxazol-5-yl]methanol
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Amberlyst 15 supported nitrosonium ion as an efficient reagent for regeneration of carbonyl compounds from oximes, hydrazones and semicarbazones.
Lakouraj MM, et al.
Reactive functional Polymers, 66(9), 910-915 (2006)
Solid-phase synthesis of 5-isoxazol-4-yl-[1,2,4] oxadiazoles.
Quan C and Kurth M.
The Journal of Organic Chemistry, 69(5), 1470-1474 (2004)
A mild and selective method for the conversion of oximes into ketones and aldehydes by the use of N-bromophthalimide.
Khazaei A, et al.
J. Chem. Res. (M), 2004(10), 695-696 (2004)
Facile and Chemoselective Microwave-Assisted Cleavage of Oximes to Their Corresponding Carbonyl Compounds Using N,N?-Dibromo-N,N?-1,3-propylene-bis[(4-methylphenyl)sulfonamide] as a Deoximating Reagent.
Khazaei A, et al.
Synthesis, 2004(17), 2784-2786 (2004)
Hypervalent iodine mediated synthesis of di-and tri-substituted isoxazoles via [3+2] cycloaddition of nitrile oxides.
Singhal A, et al.
Tetrahedron, 57(7), 719-722 (2016)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service