All Photos(1)
About This Item
Empirical Formula (Hill Notation):
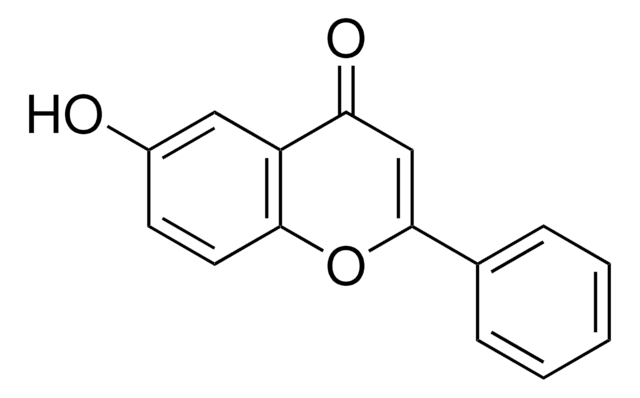
C15H12O3
CAS Number:
Molecular Weight:
240.25
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
solid
mp
220-222 °C (dec.) (lit.)
functional group
ketone
phenyl
SMILES string
Oc1ccc2OC(CC(=O)c2c1)c3ccccc3
InChI
1S/C15H12O3/c16-11-6-7-14-12(8-11)13(17)9-15(18-14)10-4-2-1-3-5-10/h1-8,15-16H,9H2
InChI key
XYHWPQUEOOBIOW-UHFFFAOYSA-N
Gene Information
rat ... Ar(24208)
Looking for similar products? Visit Product Comparison Guide
General description
6-Hydroxyflavanone is a flavonoid. Its biotransformation by Aspergillus niger strains have been described. Crystal structure of S and R enantiomers of 6-hydroxyflavanone has been reported to have four crystallographic sites of the unit cell in an approximate 3:1/1:3 ratio. It was identified as a metabolite of flavnone on incubation with rat liver microsomes by positive ion electrospray LC/MS analysis.
Application
6-Hydroxyflavanone (6-HF) has been used for the enantiomeric separation of flavonoids using polysaccharide-based chiral stationary phases by nano-liquid chromatography (nano-LC). Racemic 6-HF may be used in the synthesis of 6-propionoxy-flavanone (6-PF). 6-HF may be employed as synthetic flavone to investigate the mechanism of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) induced cytotoxic and apoptotic effects in HeLa cancer cells.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Disordered 6-hydroxyflavanone.
Bialonska A, et al.
Acta Crystallographica Section E, Structure Reports Online, 63(2), 430-431 (2007)
Ewelina Szliszka et al.
Molecules (Basel, Switzerland), 17(10), 11693-11711 (2012-10-03)
Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) is considered as the most promising anticancer agent in the TNF superfamily because of its selective cytotoxicity against tumor cells versus normal primary cells. However, as more tumor cells are reported to be resistant
Microbial transformations of flavanone and 6-hydroxyflavanone by Aspergillus niger strains.
Kostrzewa-Suslow E, et al.
Journal of Molecular Catalysis. B, Enzymatic, 39(1), 18-23 (2006)
Dejan Nikolic et al.
Drug metabolism and disposition: the biological fate of chemicals, 32(4), 387-397 (2004-03-25)
Flavonoids represent a diverse group of natural pigments widely distributed in the plant kingdom and are an important component of human diet due to their high content in fruits and vegetables. Since many flavonoids have been shown to be potent
Kahina Si-Ahmed et al.
Analytica chimica acta, 738, 85-94 (2012-07-14)
Three polysaccharide-based chiral stationary phases, Sepapak(®) 1, Sepapak(®) 2 and Sepapak(®) 3 have been evaluated in the present work for the stereoisomer separation of a group of 12 flavonoids including flavanones (flavanone, 4'-methoxyflavanone, 6-methoxyflavanone, 7-methoxyflavanone, 2'-hydroxyflavanone, 4'-hydroxyflavanone, 6-hydroxyflavanone, 7-hydroxyflavanone, hesperetin
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service