All Photos(1)
About This Item
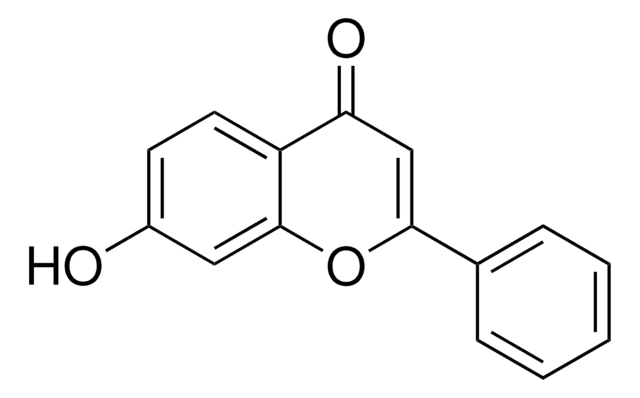
Empirical Formula (Hill Notation):
C15H10O3
CAS Number:
Molecular Weight:
238.24
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
mp
234-236 °C (lit.)
functional group
ketone
phenyl
SMILES string
Oc1ccc2OC(=CC(=O)c2c1)c3ccccc3
InChI
1S/C15H10O3/c16-11-6-7-14-12(8-11)13(17)9-15(18-14)10-4-2-1-3-5-10/h1-9,16H
InChI key
GPZYYYGYCRFPBU-UHFFFAOYSA-N
Gene Information
rat ... Ar(24208) , Gabra2(29706)
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
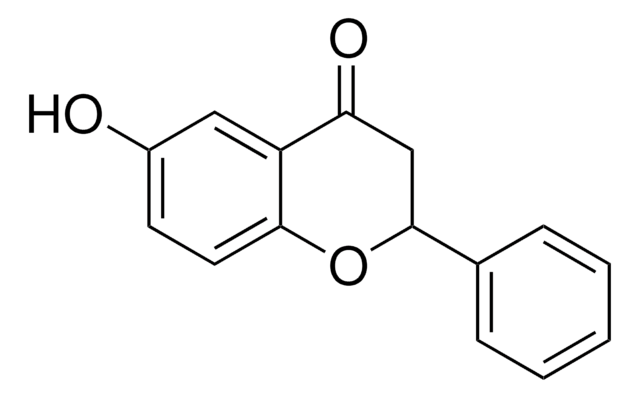
Structure of 6-hydroxyflavone.
Seetharaman J and Rajan SS.
Acta Crystallographica Section C, Structural Chemistry, 48(9), 1714-1715 (1992)
Xing Wang et al.
PloS one, 10(3), e0116409-e0116409 (2015-03-20)
Inflammatory responses by kidney mesangial cells play a critical role in the glomerulonephritis. The anti-inflammatory potential of nineteen mono-, di- and polyhydroxylated flavones including fisetin, quercetin, morin, tricetin, gossypetin, apigenin and myricetin were investigated on rat mesangial cells with lipopolysaccharide
Zia Ud Din et al.
Histology and histopathology, 35(10), 1197-1209 (2020-09-11)
In this study, the flavonoid, 6-hydroxyflavone was investigated for its renal protective activity in the cisplatin rat model of nephrotoxicity. Male Sprague-Dawley rats weighing 200-250 g were included in the study. 6-Hydroxyflavone was daily administered at 25 and 50 mg/kg
A novel and facile iodine (III)-mediated approach for C (5)-acetoxylation of 6-hydroxyflavone and 6-hydroxyflavanones.
Prakash O, et al.
Tetrahedron Letters, 45(49), 9065-9067 (2004)
Angélica Flores-Flores et al.
Drug development research, 80(2), 218-229 (2018-11-06)
Previously, we described tracheal rat rings relaxation by several flavonoids, being 6-hydroxyflavone (6-HOF) the most active derivative of the series. Thus, its mechanism of action was determined in an ex vivo tracheal rat ring bioassay. The anti-asthmatic effect was assayed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service