232807
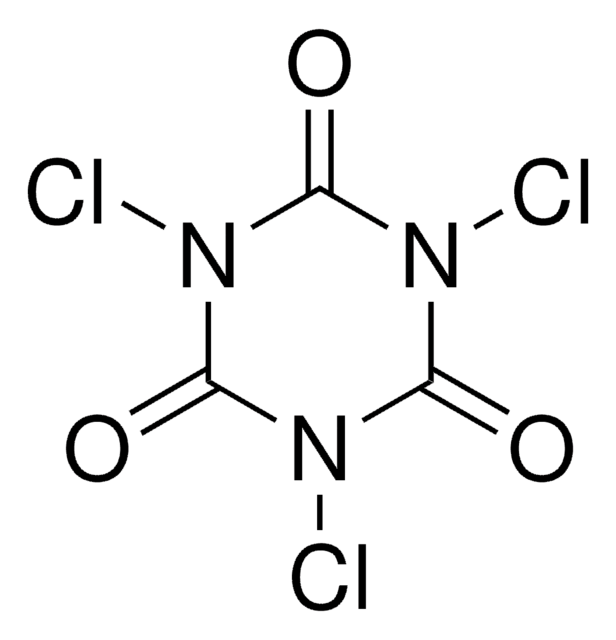
1,3-Dichloro-5,5-dimethylhydantoin
available chlorine 68 %
Synonym(s):
1,3-Dichloro-5,5-dimethyl-2,4-imidazolidinedione, DCDMH, NSC 33307, NSC 38630
About This Item
Recommended Products
form
powder
composition
available chlorine, 68%
mp
132-134 °C (lit.)
solubility
water: soluble 0.21% at 25 °C(lit.)
carbon tetrachloride: freely soluble 12.5%(lit.)
chloroform: freely soluble 14%(lit.)
methylene chloride: freely soluble 30%(lit.)
benzene: freely soluble 9.2%(lit.)
chlorinated solvents: freely soluble at 25 °C(lit.)
SMILES string
CC1(C)N(Cl)C(=O)N(Cl)C1=O
InChI
1S/C5H6Cl2N2O2/c1-5(2)3(10)8(6)4(11)9(5)7/h1-2H3
InChI key
KEQGZUUPPQEDPF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- in reaction of chlorination of cytosine base
- in the synthesis of α-chloroacetophenones
- as an effective oxidizing agent for the oxidation of urazoles and bis-urazoles to their corresponding triazolinediones
- Microwave-assisted aromatization of trisubstituted pyrazolines
- Asymmetric chlorolactonization chlorenium source
- Oxidative chlorination for the synthesis of arenesulfonyl chlorides
- Selective halogenation for the synthesis of halo ketones
- Chlorination reactions
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Ox. Sol. 2 - Skin Irrit. 2 - Skin Sens. 1
Supplementary Hazards
Storage Class Code
5.1B - Oxidizing hazardous materials
WGK
WGK 2
Flash Point(F)
345.2 °F
Flash Point(C)
174 °C
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service