156361
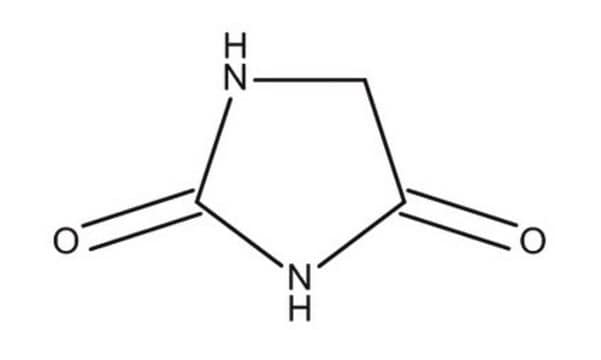
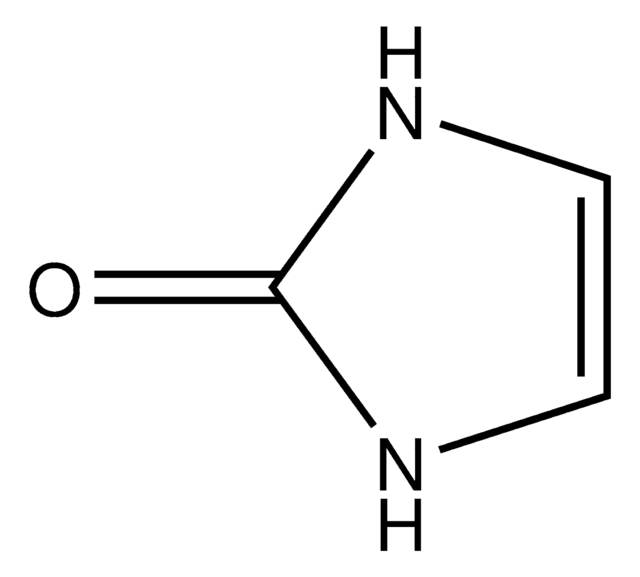
Hydantoin
98%
Synonym(s):
2,4-Imidazolidinedione, Glycolylurea
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C3H4N2O2
CAS Number:
Molecular Weight:
100.08
Beilstein:
110598
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
powder
mp
218-220 °C (lit.)
SMILES string
O=C1CNC(=O)N1
InChI
1S/C3H4N2O2/c6-2-1-4-3(7)5-2/h1H2,(H2,4,5,6,7)
InChI key
WJRBRSLFGCUECM-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Reactant for synthesis of:
N-benzyl aplysinopsin analogs as anticancer agents
D-glutamic acid based inhibitors
Antidiabetic chromonyl-2,4-thiazolidinediones
GSK-3β inhibitors with brain permeability
Thiazolidinedione derivatives as 15-PGDH inhibitors
Radio-sensitizing agents
N-benzyl aplysinopsin analogs as anticancer agents
D-glutamic acid based inhibitors
Antidiabetic chromonyl-2,4-thiazolidinediones
GSK-3β inhibitors with brain permeability
Thiazolidinedione derivatives as 15-PGDH inhibitors
Radio-sensitizing agents
The product has been used as a substrate (at 40 °C and pH 9.0) to determine the D-hydantoinase activity in adzuki bean extract.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Mohammad A Khanfar et al.
Journal of medicinal chemistry, 53(24), 8534-8545 (2010-11-19)
Dysregulation of glycogen synthase kinase (GSK-3β) is implicated in the pathophysiology of many diseases, including type-2 diabetes, stroke, Alzheimer's, and others. A multistage virtual screening strategy designed so as to overcome known caveats arising from the considerable flexibility of GSK-3β
Suguru Nishinami et al.
International journal of biological macromolecules, 114, 497-503 (2018-03-06)
Allantoin is widely used in pharmaceutical and cosmetic products, and is composed of a hydantoin ring and a ureido group. Recent reports showed that allantoin suppresses thermal aggregation of hen egg white lysozyme (LYZ). However, structural insight into the properties
Tihomir Tomasić et al.
Journal of medicinal chemistry, 54(13), 4600-4610 (2011-05-20)
MurD ligase is one of the key enzymes participating in the intracellular steps of peptidoglycan biosynthesis and constitutes a viable target in the search for novel antibacterial drugs to combat bacterial drug-resistance. We have designed, synthesized, and evaluated a new
Y Thirupathi Reddy et al.
Bioorganic & medicinal chemistry letters, 20(2), 600-602 (2009-12-17)
A series of (Z)-5-((N-benzyl-1H-indol-3-yl)methylene)imidazolidine-2,4-dione (9a-9m) and 5-((N-benzyl-1H-indol-3-yl)methylene)pyrimidine-2,4,6(1H,3H,5H)-trione (10a-10i) derivatives that incorporate a variety of aromatic substituents in both the indole and N-benzyl moieties have been synthesized. These analogs were evaluated for their radiosensitization activity against the HT-29 cell line. Three
Effect of treatment with compressed CO2 and propane on D-hydantoinase activity
Andrade JM, et al.
Journal of Supercritical Fluids, 46(2), 342-350 (2008)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service